Abstract
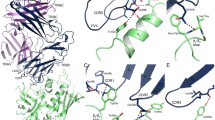
SUPERANTIGENS such as the staphylococcal enterotoxins bind to major histocompatibility complex (MHC) class II molecules and activate T cells through a specific interaction between the V/β region of the T-cell antigen receptor (TCR) and the toxin1–4. The TCR β-chain alone is sufficient to produce the interaction with the enterotoxin–class II complex5. Identification of the regions of enterotoxins that interact with TCR has so far proved equivocal because of difficulties in distinguishing between direct effects on T-cell recognition and indirect effects resulting from alteration of binding to class II. For example, amino-terminal truncations of SEB abrogated T-cell stimulation whereas carboxy-terminal truncation of SEA stopped its mitogenic activity10,11. The most comprehensive study to date, accounting for both enterotoxin binding to class II and enterotoxin interactions with the TCR, identified two functionally important regions for SEB binding to TCR12. Although the amino-acid sequences of staphylococcal enterotoxins A and E are 82% identical6, they activate T cells bearing different Vβ elements. We have assayed the binding of cells coated with these enterotoxins to soluble secreted TCR β-chain protein and find that Vβ3 binds enterotoxin A but not E, whereas Vβ11 binds enterotoxin but not A. To map the amino-acid residues responsible for these different binding specificities, we prepared a series of hybrids between the two staphylococcal enterotoxins. We report that just two amino-acid residues near the carboxy terminus of the enterotoxins are responsible for the dis-crimination between these molecules by Vβ3 and Vβ11. The crystal structure of staphylococcal enterotoxin B (ref. 12) indicates that these form part of the outer rim of the site recognizing TCR.
Similar content being viewed by others
References
Marrack, P. & Kappler, J. Science 248, 705–711 (1990).
Herman, A., Kappler, J. W., Marrack, P. & Pullen, A. M. Rev. Immun. 9, 745–772 (1991).
Johnson, H. M., Russell, J. K. & Pontzer, C. H. FASEB J. 5, 2706–2712 (1991).
Gascoigne, N. R. J. Semin. Immun. (in the press).
Gascoigne, N. R. J. & Ames, K. T. Proc. natn. Acad. Sci. U.S.A. 88, 613–616 (1991).
Couch, J. L., Soltis, M. T. & Betley, M. J. J. Bact. 170, 2954–2960 (1988).
Swaminathan, S., Furey, W., Pletcher, J. & Sax, M. Nature 359, 801–806 (1992).
Callahan, J. E., Herman, A., Kappler, J. W. & Marrack, P. J. Immun. 144, 2473–2479 (1990).
Takimoto, H. et al. Eur. J. Immun. 20, 617–621 (1990).
Buelow, R. et al. J. Immun. 148, 1–6 (1992).
Hufnagle, W. O., Tremaine, M. T. & Betley, M. J. Infect. Immun. 59, 2126–2134 (1991).
Kappler, J. W., Herman, A., Clements, J. & Marrack, P. J. exp. Med. 175, 387–396 (1992).
Singh, B. R. & Betley, M. J. J. biol. Chem. 264, 4404–4411 (1989).
Gascoigne, N. R. J. J. biol. Chem. 265, 9296–9301 (1990).
Vacchio, M. S., Kanagawa, O., Tomonari, K. & Hodes, R. J. J. exp. Med. 175, 1405–1408 (1992).
Smith, H. P., Nguyen, P., Woodland, D. L. & Blackman, M. A. J. Immun. 149, 887–896 (1992).
Fraser, J. D. Nature 339, 221–223 (1989).
Bill, J., Kanagawa, O., Woodland, D. L. & Palmer, E. J. exp. Med. 169, 1405–1419 (1989).
Pullen, A. M., Marrack, P. & Kappler, J. W. Nature 335, 796–801 (1988).
Kanagawa, O., Palmer, E. & Bill, J. Cell. Immun. 119, 412–426 (1989).
Kubo, R. T., Born, W., Kappler, J. W., Marrack, P. & Pigeon, M. J. Immun. 142, 2736–2742 (1989).
Palmer, M. S., Bentley, A., Gould, K. & Townsend, A. R. M. Nucleic Acids Res. 17, 2353 (1989).
Summers, M. D. & Smith, G. E. A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures (Texas Agricultural Experiment Station, Bull. No. 1555, College Station, Texas 77843, 1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Irwin, M., Hudson, K., Fraser, J. et al. Enterotoxin residues determining T-cell receptor Vβ binding specificity. Nature 359, 841–843 (1992). https://doi.org/10.1038/359841a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/359841a0
- Springer Nature Limited
This article is cited by
-
Human TGFalpha-derived peptide TGFalphaL3 fused with superantigen for immunotherapy of EGFR-expressing tumours
BMC Biotechnology (2010)
-
Differential diagnosis of eosinophilic chronic rhinosinusitis
Current Allergy and Asthma Reports (2006)
-
Residues defining Vβ specificity in staphylococcal enterotoxins
Nature Structural Biology (1995)
-
Crystal and solution structures of superantigenic staphylococcal enterotoxins compared
Nature Structural & Molecular Biology (1994)
-
Structural basis of superantigen action inferred from crystal structure of toxic-shock syndrome toxin-1
Nature (1994)