Abstract
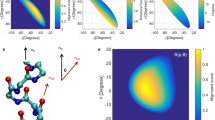
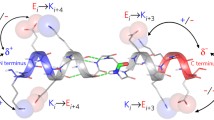
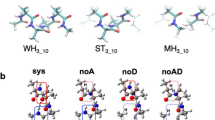
SHORT alanine peptides, containing 16 or 17 residues, appear to form α-helices in aqueous solution1–4. But the main spectroscopic analyses used on helical peptides (circular dichroism5 and nuclear magnetic resonance6–8) cannot distinguish between an α-helix (in which the ith residue is hydrogen-bonded to residue i+4; ref. 9) and the next most common peptide helix, the 310-helix10 (i → i + 3 hydrogen-bonding). To address this problem we have designed single and doubly spin-labelled analogues of alanine-based peptides in which the nitroxide spin label forms an unbranched side chain extending from the sulphur atom of a cysteine residue. Here we report the circular dichroism, Fourier-transform infrared and electron-spin resonance spectra of these peptides under helix-forming conditions. The infrared absorbance gives an amide I' band with a frequency that is substantially different from that observed for α-helices. The electron-spin resonance spectra of doubly labelled helices show that the ranking of distances between side chains, around a single turn (residues 4–8), is inconsistent with an α-helical structure. Our experiments suggest that the more likely peptide geometry is a 310-helix.
Similar content being viewed by others
References
Marqusee, S., Robbins, V. H. & Baldwin, R. L. Proc. natn. Acad. Sci. U.S.A. 86, 5286–5290 (1989).
Merutka, G., Lipton, W., Shalongo, W., Park, S. H. & Stellwagen, E. Biochemistry 29, 7511–7515 (1990).
Chakrabartty, A., Schellman, J. A. & Baldwin, R. L. Nature 351, 586–588 (1991).
Padmanabhan, S., Marqusee, S., Ridgeway, T., Laue, T. M. & Baldwin, R. L. Nature 344, 268–270 (1990).
Manning, M. C. & Woody, R. W. Biopolymers 31, 569–586 (1991).
Osterhout, J. J. et al. Biochemistry 28, 7059–7064 (1989).
Bradley, E. K., Thomason, J. F., Cohen, F. E., Kosen, P. A. & Kuntz, I. D. J. molec. Biol. 215, 607–622 (1990).
Würthrich, K. NMR of Proteins and Nucleic Acids (Wiley, New York, 1986).
Voet, D. & Voet, J. G. Biochemistry (Wiley, New York, 1990).
Barlow, D. J. & Thornton, J. M. J. molec. Biol. 201, 601–619 (1988).
Todd, A. P. & Millhauser, G. L. Biochemistry 30, 5515–5523 (1991).
Miick, S. M., Rodd, A. P. & Millhauser, G. L. Biochemistry 30, 9498–9503 (1991).
Byler, D. M. & Susi, H. Biopolymers 25, 469–487 (1986).
Kennedy, D. F., Crisma, M., Toniolo, C. & Chapman, D. Biochemistry 30, 6541–6548 (1991).
Malcolm, B. R. Biopolymers 22, 319–321 (1983).
Dwivedi, A. M., Krimm, S. & Malcolm, B. R. Biopolymers 23, 2025–2065 (1984).
Prestrelski, S. J., Byler, D. M. & Thompson, M. P. Int. J. Peptide Protein Res. 37, 508–512 (1991).
Luckhurst, G. R. in Spin labeling: Theory and Applications (ed. Berliner, L. J.) Ch. 4 (Academic, New York, 1976).
Falle, H. R. et al. Molec. Phys. 11, 49–56 (1966).
Lemaire, H., Rassat, A., Rey, P. & Luckhurst, G. R. Molec. Phys. 14, 441–447 (1968).
Krystek, S. R. et al. FEBS Lett. 299, 255–261 (1992).
Toniolo, C. & Benedetti, E. Trends. biochem. Sci. 16, 350–353 (1991).
Karle, I. L. & Balaram, P. Biochemistry 29, 6747–6756 (1990).
Gautam, B., Bagchi, K. & Kuki, A. Biopolymers 31, 1763–1774 (1991).
Tirado-Rives, J. & Jorgensen, W. L. Biochemistry 30, 3864–3871 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miick, S., Martinez, G., Fiori, W. et al. Short alanine-based peptides may form 310-helices and not α-helices in aqueous solution. Nature 359, 653–655 (1992). https://doi.org/10.1038/359653a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/359653a0
- Springer Nature Limited
This article is cited by
-
Constructing a structural model of troponin using site-directed spin labeling: EPR and PRE-NMR
Biophysical Reviews (2019)
-
Thermodynamics of helix formation in small peptides of varying length in vacuo, in implicit solvent, and in explicit solvent
Journal of Molecular Modeling (2019)
-
Peptide Folding Problem: A Molecular Dynamics Study on Polyalanines Using Different Force Fields
International Journal of Peptide Research and Therapeutics (2013)
-
The spin label amino acid TOAC and its uses in studies of peptides: chemical, physicochemical, spectroscopic, and conformational aspects
Biophysical Reviews (2012)
-
Helix and H-bond formations of alanine-based peptides containing basic amino acids
Structural Chemistry (2011)