Abstract
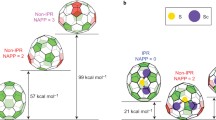
MACROSCOPIC amounts of the two fullerenes C60 and C70 have been available for a year1, and have already had an enormous impact on research in chemistry and physics. Experimentalists are now turning their attention to the higher fullerenes2,3. Qualitative molecular-orbital theory predicts4–6 stability for Cn with n = 60, 70, (72), 76, 78,84,..., of which all but C72 have now been produced by evaporation of graphite1,3, and in general for infinite series of closed-shell neutral fullerenes for n=60 + 6k (k -£ 1), 70 + 30k, 84 + 36k (all k)7–9. Recent experimental observations of endohedral LaCn metallofullerenes10 have been rationalized in terms of 'magic numbers' for fulleride anions C2n, for which special stability is predicted11 at n =74, 82, 88,... ; but the exact extent of charge transfer in these complexes has yet to be determined. Here we present calculations of magic numbers in the fullerenium sequence C2+n (n =74,80, (88), 92) and show that the electron count determines stability and the atom count determines structure in all three (neutral, anionic and cationic) series. Stable cations have two carbons more, and stable anions two carbons less, than the corresponding stable neutral cluster. We predict likely structures of the 'magic' cations.
Similar content being viewed by others
References
Krätschmer, K., Lamb, L. D., Fostiropoulos, K. & Huffman, D. R. Nature 347, 354–358 (1990).
Diederich, F. et al. Science 252, 548–551 (1991).
Ettl, R., Chao, I., Diederich, F. & Whetten, R. L. Nature 353, 149–153 (1991).
Manolopoulos, D. E. J. chem. Soc. Faraday Trans. 87, 2861–2862 (1991).
Fowler, P. W., Manolopoulos, D. E. & Batten, R. C. J. chem. Soc. Faraday Trans. 87, 3103–3104 (1991).
Fowler, P. W. J. chem. Soc. Faraday Trans. 87, 1945–1946 (1991).
Fowler, P. W. & Steer, J. I. J. chem. Soc. Chem. Commun. 1403–1405 (1987).
Fowler, P. W., Cremona, J. E. & Steer, J. I. Theor. Chim Acta. 73, 1–26 (1988).
Fowler, P. W. J. chem. Soc. Faraday Trans. 86, 2073–2077 (1990).
Chai, Y. et al. J. phys. Chem. (in the press).
Manolopoulos, D. E. & Fowler, P. W. Chem. Phys. Lett. 187, 1–7 (1991).
Haufler, R. E. et al. Mat. Res. Soc. Proc. 206, 627–638 (1991).
Kroto, H. W. Nature 329, 529–531 (1987).
Manolopoulos, D. E., May, J. C. & Down, S. E. Chem. Phys. Lett. 181, 105–111 (1991).
Diederich, F. et al. Science (in the press).
Rosen, A. & Wastberg, B. J. Am. chem. Soc. 110, 8701–8703 (1988).
Chang, A. H. H., Ermler, W. C. & Pitzer, R. M. J. chem. Phys. 94, 5004–5010 (1991).
Johnson, R. D., de Vries, M. S., Salem, J., Bethune, D. S. & Yannoni, C. S. Nature (in the press).
Weaver, J. H. et al. Chem. Phys. Lett. (in the press).
Fowler, P. W. Chem. Phys. Lett. 131, 444–450 (1986).
de Heer, W. A., Knight, W. D., Chou, M. Y. & Cohen, M. L. Solid State Phys. 40, 93–181 (1981).
Stone, A. J. Inorg. Chem. 20, 563–571 (1981).
Wade, K. J. chem. Soc. Chem. Commun. 792–793 (1971).
Holloway, J. H. et al. J. chem. Soc. Chem. Commun. 966–969 (1991).
Guo, T., Jin, C. & Smalley, R. E. J. phys. Chem. 95, 4948–4950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fowler, P., Manolopoulos, D. Magic numbers and stable structures for fullerenes, fullerides and fullerenium ions. Nature 355, 428–430 (1992). https://doi.org/10.1038/355428a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/355428a0
- Springer Nature Limited
This article is cited by
-
Cage connectivity and frontier π orbitals govern the relative stability of charged fullerene isomers
Nature Chemistry (2015)
-
Atoms in carbon cages: the structure and properties of endohedral fullerenes
Nature (1993)
-
Electronic and geometric structure of C72
Zeitschrift f�r Physik D Atoms, Molecules and Clusters (1993)
-
Generating functions for the cage isomers of the C20n icosahedral fullerenes
Journal of Mathematical Chemistry (1993)