Abstract
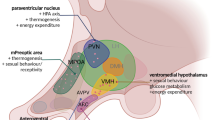
The administration of leptin1 to leptin-deficient humans, and the analogous Lepob/Lepob mice, effectively reduces hyperphagia and obesity2,3. But common obesity is associated with elevated leptin, which suggests that obese humans are resistant to this adipocyte hormone. In addition to regulating long-term energy balance, leptin also rapidly affects neuronal activity4,5,6. Proopiomelanocortin (POMC) and neuropeptide-Y types of neurons in the arcuate nucleus of the hypothalamus7 are both principal sites of leptin receptor expression and the source of potent neuropeptide modulators, melanocortins and neuropeptide Y, which exert opposing effects on feeding and metabolism8,9. These neurons are therefore ideal for characterizing leptin action and the mechanism of leptin resistance; however, their diffuse distribution makes them difficult to study. Here we report electrophysiological recordings on POMC neurons, which we identified by targeted expression of green fluorescent protein in transgenic mice. Leptin increases the frequency of action potentials in the anorexigenic POMC neurons by two mechanisms: depolarization through a nonspecific cation channel; and reduced inhibition by local orexigenic neuropeptide-Y/GABA (γ-aminobutyric acid) neurons. Furthermore, we show that melanocortin peptides have an autoinhibitory effect on this circuit. On the basis of our results, we propose an integrated model of leptin action and neuronal architecture in the arcuate nucleus of the hypothalamus.




Similar content being viewed by others
References
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994); erratum ibid. 374, 479 (1995).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Campfield, L. A., Smith, F. J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 (1995).
Kim, M. S. et al. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J. Clin. Invest. 105, 1005–1011 (2000).
Haynes, W. G., Morgan, D. A., Walsh, S. A., Mark, A. L. & Sivitz, W. I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100, 270–278 (1997).
Haynes, W. G., Morgan, D. A., Djalali, A., Sivitz, W. I. & Mark, A. L. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33, 542–547 (1999).
Hakansson, M. L., Brown, H., Ghilardi, N., Skoda, R. C. & Meister, B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 18, 559–572 (1998).
Kalra, S. P. et al. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20, 68–100 (1999).
Cone, R. D. The central melanocortin system and energy homeostasis. Trends Endocrinol. Metab. 10, 211–216 (1999).
Elias, C. F. et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23, 775–786 (1999).
Glaum, S. R. et al. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Mol. Pharmacol. 50, 230–235 (1996).
Spanswick, D., Smith, M. A., Groppi, V. E., Logan, S. D. & Ashford, M. L. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390, 521–525 (1997).
Lee, K., Dixon, A. K., Richardson, P. J. & Pinnock, R. D. Glucose-receptive neurones in the rat ventromedial hypothalamus express KATP channels composed of Kir6.1 and SUR1 subunits. J. Physiol. (Lond.) 515, 439–452 (1999).
Shiraishi, T., Sasaki, K., Niijima, A. & Oomura, Y. Leptin effects on feeding-related hypothalamic and peripheral neuronal activities in normal and obese rats. Nutrition 15, 576–579 (1999).
Bagnol, D. et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. [online] (cited 25 Aug. 99) <http://www.jneurosci.org/cgi/content/full/19/18/RC26> (1999).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000).
Young, J. I. et al. Authentic cell-specific and developmentally regulated expression of pro-opiomelanocortin genomic fragments in hypothalamic and hindbrain neurons of transgenic mice. J. Neurosci. 18, 6631–6640 (1998).
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego, 1997).
Kelly, M. J., Loose, M. D. & Ronnekleiv, O. K. Opioids hyperpolarize β-endorphin neurons via µ-receptor activation of a potassium conductance. Neuroendocrinology 52, 268–275 (1990).
Slugg, R. M., Hayward, M. D., Ronnekleiv, O. K., Low, M. J. & Kelly, M. J. Effect of the µ-opioid agonist DAMGO on medial basal hypothalamic neurons in β-endorphin knock-out mice. Neuroendocrinology 72, 208–217 (2000).
Powis, J. E., Bains, J. S. & Ferguson, A. V. Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am. J. Physiol. 274, R1468–R1472 (1998).
Horvath, T. L., Bechmann, I., Naftolin, F., Kalra, S. P. & Leranth, C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 756, 283–286 (1997).
Broberger, C., Landry, M., Wong, H., Walsh, J. N. & Hokfelt, T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology 66, 393–408 (1997).
King, P. J., Widdowson, P. S., Doods, H. N. & Williams, G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J. Neurochem. 73, 641–646 (1999).
Grieco, P., Balse, P. M., Weinberg, D., MacNeil, T. & Hruby, V. J. d-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. J. Med. Chem. 43, 4998–5002 (2000).
Csiffary, A., Gorcs, T. J. & Palkovits, M. Neuropeptide Y innervation of ACTH-immunoreactive neurons in the arcuate nucleus of rats: a correlated light and electron microscopic double immunolabeling study. Brain Res. 506, 215–222 (1990).
Horvath, T. L., Naftolin, F. & Leranth, C. GABAergic and catecholaminergic innervation of mediobasal hypothalamic beta-endorphin cells projecting to the medial preoptic area. Neuroscience 51, 391–399 (1992).
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163 (1999).
Halaas, J. L. et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl Acad. Sci. USA 94, 8878–8883 (1997).
Acknowledgements
We wish to thank V. J. Hruby for the d-Trp8-γMSH, O. K. Ronnekliev, R. G. Allen and M. R. Brown for antisera and J. T. Williams and J. M. Brundege for advice. This work was supported by the NIH, a Fogarty International Research Collaborative Award, the International Scholar Program of the Howard Hughes Medical Institute, and Agencia Nacional de Promoción Cientifica y Technológica.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cowley, M., Smart, J., Rubinstein, M. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001). https://doi.org/10.1038/35078085
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35078085
- Springer Nature Limited
This article is cited by
-
Genetic labeling reveals spatial and cellular expression pattern of neuregulin 1 in mouse brain
Cell & Bioscience (2023)
-
Effect of acute and long-term exercise on leptin levels in depressed outpatients
BMC Public Health (2023)
-
Metabolic and feeding adjustments during pregnancy
Nature Reviews Endocrinology (2023)
-
The melanocortin action is biased toward protection from weight loss in mice
Nature Communications (2023)
-
Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions
Molecular Psychiatry (2023)