Abstract
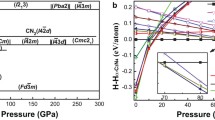
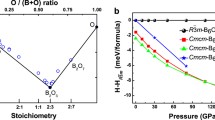
A material as hard as diamond or cubic boron nitride has yet to be identified1,2,3,4,5,6, but here we report the discovery of a cotunnite-structured titanium oxide which represents the hardest oxide known. This is a new polymorph of titanium dioxide, where titanium is nine-coordinated to oxygen in the cotunnite (PbCl2) structure. The phase is synthesized at pressures above 60 gigapascals (GPa) and temperatures above 1,000 K and is one of the least compressible and hardest polycrystalline materials to be described.

Similar content being viewed by others
References
Cohen, M. L. Solid State Commun. 92, 45–52 (1994).
Riedel, R. Adv. Mater. 6, 549–556 (1994).
Sung, C. M. & Sung, M. Mater. Chem. Phys. 43, 1–6 (1996).
Léger, J. M., Haines, J. & Blanzat, B. J. Mater. Sci. Lett. 13, 1688–1692 (1994).
Lundin, U. et al. Phys. Rev. B 57, 4979–4982 (1998).
Desgreniers, S. & Lagarec, K. Phys. Rev. B 59, 8467–8472 (1999).
Léger, J. M. Nature 383, 401 (1996).
Teter, D. M. Mater. Res. Sci. Bull. 22–27 (1998).
Jhi, S. H. et al. Nature 399, 132–134 (1999).
Haines, J. & Léger, J. M. Physica B 192, 232–239 (1993).
Olsen, J. S., Gerward, L. & Jiang, J. Z. J. Phys. Chem. Solids 60, 229–233 (1999).
Larson, A. C. & Von Dreele, R. B. Los Alamos National Laboratory, LAUR, 86 (1994).
Leinenweber, K., Schuelke, U., Ekundit, S. & McMillan, P. F. in Properties of Earth and Planetary Materials at High Pressure and Temperature (eds Manghnani, M. H. & Yagi, T.) 101, 97–105 (Am. Geophys.Union, Geophys. Monogr., Washington, 1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubrovinsky, L., Dubrovinskaia, N., Swamy, V. et al. The hardest known oxide. Nature 410, 653–654 (2001). https://doi.org/10.1038/35070650
Issue Date:
DOI: https://doi.org/10.1038/35070650
- Springer Nature Limited
This article is cited by
-
Decomposition of Nb3Si and mechanical-property improvement by adding appropriate amount of MgO in Nb–16Si–20Ti alloy
Rare Metals (2023)
-
Theoretical studies for stability, mechanical properties, electronic properties and Debye temperature of novel Cr2C structures
Journal of Materials Science (2022)
-
Characterisation of oxygen defects and nitrogen impurities in TiO2 photocatalysts using variable-temperature X-ray powder diffraction
Nature Communications (2021)
-
One-step electrochemical deposition of thin film titanium suboxide in basic titanyl sulfate solution at room temperature
Journal of Solid State Electrochemistry (2020)
-
Photocatalytic Activity, Microstructures and Luminescent Study of Ti-ZS:M Nano-composites Materials
Journal of Inorganic and Organometallic Polymers and Materials (2020)