Abstract
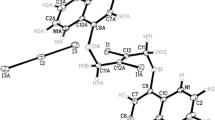
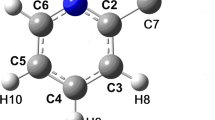
THEinteraction of water with aromatic moieties is of importance in biological systems, as most encounter an aqueous environment during their normal functions. For example, common constituents of globular proteins such as phenylalanine, tryptophan and tyrosine possess aromatic side-chains1 that may encounter water molecules inside the protein structure2. As a model for hydrogen-bonding interactions with aromatics, we have performed X-ray diffraction studies on crystalline Na4[calix[4]arene sulphonate].13.5H2O. Calixarene molecules3,4contain hydrophobic cavities comprised of aromatic groups, rimmed, in the case of the water-soluble sulphonates (R = -SO3Na), by hydrophilic groups5,6. In the absence of a hydrophobic guest, the cavity invariably contains a water molecule. The low-temperature X-ray crystal structure of this compound (see Table 1 and Fig. 1) shows direct evidence for hydrogen bonding between water and the aromatic π electrons in the solid state.
Similar content being viewed by others
References
Burley, S. K. & Petsko, G. A. Science 229, 23–35 (1985).
Tuchsen, E., Hayes, J. M., Ramaprasad, S., Copie, V. & Woodward, C. Biochemistry 26, 5163–5172 (1987).
Gutsche, C. D. Calixarenes (Royal Society of Chemistry, Cambridge, 1989).
Vicens, J. & Bohmer, V. (eds) Calixarenes (Kluwer, Dordrecht, 1990).
Shinkai, S. Pure appl. Chem. 58, 1523–1530 (1986).
Coleman, A. W. et al. Angew. Chem. int. Ed. Engl. 27, 1361–1362 (1988).
Cheney, B. V., Schulz, M. W., Cheney, J. & Richards, W. G. J. Am. chem. Soc. 110, 4195–4198 (1988).
Bredas, J. L. & Street, G. B. J. Am. chem. Soc. 110, 7001–7005 (1988).
Karlstrom, G., Linse, P., Wallqvist, A. & Jonsson, B. J. Am. chem. Soc. 105, 3777–3782 (1983).
Bredas, J. L. & Street, G. B. J. chem. Phys. 90, 7291–7299 (1989).
Read, W. G., Campbell, E. J., Henderson, G. & Flygare, W. H. J. Am. chem. Soc. 103, 7670–7672 (1981).
Read, W. G., Campbell, E. J. & Henderson, G. J. chem. Phys. 78, 3501–3508 (1983).
Hamilton, W. C. & Ibers, J. A. Hydrogen Bonding in Solids 16 (Benjamin, New York, 1968).
Engdahl, A. & Nelander, B. J. phys. Chem. 89, 2860–2864 (1985).
Wanna, J., Menapace, J. A. & Bernstein, E. R. J. chem. Phys. 85, 1795–1805 (1986).
Jorgensen, W. L. & Severance, D. L. J. Am. chem. Soc. 112, 4768–4774 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atwood, J., Hamada, F., Robinson, K. et al. X-ray diffraction evidence for aromatic π hydrogen bonding to water. Nature 349, 683–684 (1991). https://doi.org/10.1038/349683a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/349683a0
- Springer Nature Limited
This article is cited by
-
Strong structuring arising from weak cooperative O-H···π and C-H···O hydrogen bonding in benzene-methanol solution
Nature Communications (2023)
-
Complexes Based on Calix[4]arene Sulfonic Acid with Acetic Acid and Its Derivatives: NMR Analysis
Applied Magnetic Resonance (2019)
-
Aspects of proton transport in calix[6]arene sulfonic acid
Ionics (2017)
-
Thiacalixarenes: emergent supramolecules in crystal engineering and molecular recognition
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2016)
-
Weakly hydrated surfaces and the binding interactions of small biological solutes
European Biophysics Journal (2012)