Abstract
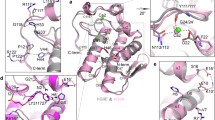
PHOSPHOLIPASES A2 play a part in a number of physiologically important cellular processes such as inflammation, blood platelet aggregation and acute hypersensitivity1,2. These processes are all initiated by the release of arachidonic acid from cell membranes which is catalysed by intracellular phospholipases A2 and followed by conversion of arachidonic acid to prostaglandins, leukotrienes or thromboxanes3. An imbalance in the production of these com-pounds can lead to chronic inflammatory diseases such as rheumatoid arthritis and asthma. Inhibitors of phospholipase A2 might therefore act to reduce the effects of inflammation, so structural information about the binding of phospholipase A2 to its substrates could be helpful in the design of therapeutic drugs. The three-dimensional structure is not known for any intracellular phospholipase A2, but these enzymes share significant sequence homology4–6 with secreted phospholipases, for which some of the structures have been determined7–10. Here we report the structure of a complex between an extracellular phospholipase A2 and a competitively inhibiting substrate analogue, which reveals considerable detail about the interaction and suggests a mechanism for catalysis by this enzyme.
Similar content being viewed by others
References
Hirata, F. & Axelrod, J. Science 209, 1082–1090 (1980).
Vadas, P. & Pruzanski, W. Lab. Invest. 4, 391–404 (1986).
Flower, R. Tr. pharmacol. Sci. 2, 186–188 (1981).
Seilhamer, J. J. et al. J. biol. Chem. 264, 5335–5338 (1989).
Kramer, R. M. et al. J. biol. Chem. 264, 5768–5775 (1989).
Mizushima, H. et al. J. Biochem. 105, 520–525 (1989).
Dijkstra, B. W., Kalk, K. H., Hol, W. G. J. & Drenth, J. J. molec. Biol. 147, 93–123 (1981).
Dijkstra, B. W., Renetseder, R., Kalk, K. H., Hol, W. G. J. & Drenth, J. J. molec. Biol. 168, 163–179 (1983).
Brunie, S., Bolin, J., Gewirth, P. & Sigler, P. B. J. biol. Chem. 260, 9742–9747 (1985).
Renetseder, R., Brunie, S., Dijkstra, B. W., Drenth, J. & Sigler, P. B. J. biol. Chem. 260, 11627–11634 (1985).
de Haas, G. H., Dijkman, R., van Oort, M. G. & Verger, R. Biochim. biophys. Acta 1043, 75–82 (1990).
Kuipers, O. P. et al. Science 244, 82–85 (1989).
Kuipers, O. P., Dijkman, R., Pals, C. E. G. M., Verheij, H. M. & de Haas, G. H. Protein Engng 2, 467–471 (1989).
Hitchcock, P. B., Mason, R., Thomas, K. M. & Shipley, G. G. Proc. natn. Acad. Sci. U.S.A. 71, 3036–3040 (1974).
Seelig, J. & Browning, J. L. FEBS Lett. 92, 41–44 (1978).
Dijkstra, B. W., Kalk, K. H. & Drenth, J. Nature 289, 604–606 (1981).
Verheij, H. M. et al. Biochemistry 19, 743–750 (1980).
Volwerk, J. J., Dedieu, A. G. R., Verheij, H. M., Dijkman, R. & de Haas, G. H. Reel. Trav. Chim. Pays-Bas 98, 214–220 (1979).
Carter, P., Bedouelle, H. & Winter, G. Nucleic Acids Res. 13, 4431–4443 (1985).
Kramer, W. et al. Nucleic Acids Res. 12, 9441–9456 (1984).
Sanger, F., Nicklen, S. & Coulson, A. R. Proc. natn. Acad. Sci. U.S.A. 74, 5463–5467 (1977).
Dijkman, R., Dekker, N. & de Haas, G. H. Biochim. biophys. Acta 1043, 67–74 (1990).
Read, R. Acta crystallogr. Sect. A 42, 140–149 (1986).
Crowther, R. A. in The Molecular Replacement Method (ed. Rossman, M. G.) 173–178 (Gordon & Breach, New York, 1972).
Crowther, R. A. & Blow, D. M. Acta crystallogr. 23, 544–548 (1967).
Tronrud, D. E., Ten Eyck, L. F. & Matthews, B. W. Acta crystallogr. Sect. A 43, 489–501 (1987).
Jones, T. A. Meth. Enzym. 115, 157–171 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thunnissen, M., AB, E., Kalk , K. et al. X-ray structure of phospholipase A2 complexed with a substrate-derived inhibitor. Nature 347, 689–691 (1990). https://doi.org/10.1038/347689a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/347689a0
- Springer Nature Limited
This article is cited by
-
Orientation and conformation of lipids in crystals of transmembrane proteins
European Biophysics Journal (2013)
-
Activation and inhibition of lipoprotein lipase in mixed monolayers of medium or long chain-triglycerides and phospholipids
Colloid & Polymer Science (1997)
-
Sterol and steryl ester regulation of phospholipase A2 from the mosquito parasiteLagenidium giganteum
Lipids (1996)
-
A twist in the tale of lipolytic enzymes
Nature Structural & Molecular Biology (1995)
-
Drug or tool, design or serendipity?
Nature Structural & Molecular Biology (1995)