Abstract
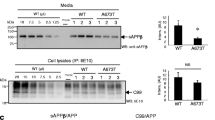
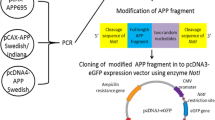
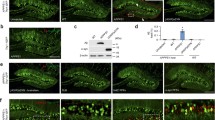
A PATHOLOGICAL hallmark of Alzheimer's disease is the deposition of amyloid fibrils in the brain. The principal component of the amyloid fibril is β/A4 protein1,2, which is derived from a large membrane-bound glycoprotein, Alzheimer amyloid protein precursor (APP)3. Although the deposition of amyloid is thought to result from the aberrant processing of APP, the detailed molecular mechanisms of amyloidogenesis remain unclear. A C-terminal fragment of APP which spans the β/A4 and cytoplasmic domains has a tendency to self-aggregate4,5. In an attempt to establish a cultured-cell model for amyloid fibril formation, we have trans-fected COS-1 cells with complementary DNA encoding the C-terminal 100 residues of APP. In the perinuclear regions of a small population of DNA-transfected cells, we observed inclusion-like deposits which showed a strong immunohistochemical reaction towards an anti-C-terminal APP antibody or an anti-β/A4 amyloid core-specific antibody. Electron microscope observations of the inclusion-carrying cells revealed an accumulation of amyloid-like fibrils of 8–22 nm diameter near and on the nuclear membrane. The fibrils showed a beaded or helical structure, and reacted positively with the anti-C-terminus antibody by immunoelectron microscopy. These results suggest that the formation of amyloid fibrils is an inherent characteristic of the C-terminal peptide of APP. The present system provides a suitable model for the molecular dissection of the process of brain amyloidogenesis.
Similar content being viewed by others
References
Glenner, G. G. & Wong, C. W. Biochem. biophys. Res. Commun. 122, 1131–1135 (1984).
Masters, C. L. et al. Proc. natn. Acad. Sci. U.S.A. 82, 4245–4249 (1985).
Kang, J. et al. Nature 325, 733–736 (1987).
Dyrks, T. et al. EMBO J. 7, 949–957 (1988).
Wolf, D. et al. EMBO J. 9, 2079–2084 (1990).
Yoshikawa, K., Williams, C. & Sabol, S. L. J. biol. Chem. 259, 14301–14308 (1984).
Wong, G. G. et al. Science 228, 810–815 (1985).
Ishii, T., Kametani, F., Haga, S. & Sato, M. Neuropath. appl. Neurobiol. 15, 135–147 (1989).
Kidd, M. Brain 87, 307–320 (1964).
Terry, R. D., Gonatas, N. K. & Weiss, M. Am. J. Pathol. 44, 269–283 (1964).
Merz, P. A. et al. Acta neuropathol. 60, 113–124 (1983).
Miyakawa, T., Katsuragi, S., Watanabe, K., Shimoji, A. & Ikeuchi, Y. Acta neuropathol. 70, 202–208 (1986).
Miyakawa, T., Watanabe, K. & Katsuragi, S. Virchows Arch. 52, 99–106 (1986).
Kirschner, D. A. et al. Proc. natn. Acad. Sci. U.S.A. 84, 6953–6957 (1987).
Sisodia, S. S., Koo, E. H., Beyreuther, K., Unterbeck, A. & Price, D. L. Science 248, 492–495 (1990).
Esch, F. S. et al. Science 248, 1122–1124 (1990).
Yankner, B. A. et al. Science 245, 417–429 (1989).
Selkoe, D. J. et al. Proc. natn. Acad. Sci. U.S.A. 85, 7341–7345 (1988).
Weidemann, A. et al. Cell 57, 115–126 (1989).
Saitoh, T. et al. Cell 58, 615–622 (1989).
Marotta, C. A. et al. Proc. natn. Acad. Sci. U.S.A. 86, 337–341 (1989).
Robakis, N. K., Ramakrishna, N., Wolfe, G. & Wisniewski, H. M. Proc. natn. Acad. Sci. U.S.A. 84, 4190–4194 (1987).
Kunkel, T. A. Proc. natn. Acad. Sci. U.S.A. 82, 488–492 (1988).
Brandl, C. J., deLeon, S., Martin, D. R. & MacLennan, D. H. J. biol. Chem. 262, 3768–3774 (1987).
Sanger, F., Nicklen, S. & Coulson, A. R. Proc. natn. Acad. Sci. U.S.A. 74, 5463–5467 (1977).
Sompayrac, L. M. & Danna, K. J. Proc. natn. Acad. Sci. U.S.A. 78, 7575–7578 (1981).
Maruyama, K. & MacLennan, D. H. Proc. natn. Acad. Sci. U.S.A. 85, 3314–3318 (1988).
Laemmli, U. K. Nature 227, 680–685 (1970).
Springer, T. A. J. biol. Chem. 256, 3833–3839 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maruyama, K., Terakado, K., Usami, M. et al. Formation of amyloid-like fibrils in COS cells overexpressing part of the Alzheimer amyloid protein precursor. Nature 347, 566–569 (1990). https://doi.org/10.1038/347566a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/347566a0
- Springer Nature Limited
This article is cited by
-
Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology
Acta Neuropathologica (2016)
-
Cell death induced by a caspase-cleaved transmembrane fragment of the Alzheimer amyloid precursor protein
Cell Death & Differentiation (2002)
-
The Alzheimer's Aβ peptide induces neurodegeneration and apoptotic cell death in transgenic mice
Nature Genetics (1995)
-
Neurotoxicity of β-amyloid
Nature (1993)
-
Abnormal distribution of cathepsin proteinases and endogenous inhibitors (cystatins) in the hippocampus of patients with Alzheimer's disease, parkinsonism-dementia complex on Guam, and senile dementia and in the aged
Virchows Archiv A Pathological Anatomy and Histopathology (1993)