Abstract
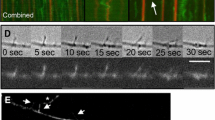
THE cytoskeleton has an important role in the generation and maintenance of the structure of the axon1,2. Microtubules, neurofilaments and actin, together with various kinds of associated proteins, form highly organized dynamic cytoskeletal structures2–4. Because tubulin and actin molecules are essential cytoskeletal components2 and are transported down the axon5,6, it is important to understand their dynamic behaviour within the axon. Although previous pulse-labelling studies have indicated that the axonal cytoskeleton is a static complex travelling down the axon6, this view has been challenged by the results of several recent experiments7–14. We have now addressed this question by analysing the recovery of fluorescence after photobleaching fluorescent analogues of tubulin and actin in the axons of cultured neurons. We did not observe movement or spreading of bleached zones along the axon, both in neurons injected with fluorescein-labelled tubulin and actin. All bleached zones recovered their fluorescence gradually, however, indicating that microtubules and actin filaments are not static polymers moving forward within the axon, but are dynamic structures that continue to assemble along the length of the axon.
Similar content being viewed by others
References
Mitchison, T. & Kirschner, M. Neuron 1, 761–772 (1988).
Hirokawa, N. J. Cell Biol. 94, 129–142 (1982).
Hirokawa, N., Glicksman, M. A. & Willard, M. B. J. Cell Biol. 98, 1523–1536 (1984).
Hirokawa, N., Bloom, G. S. & Vallee, R. B. J. Cell Biol. 101, 227–239 (1985).
Willard, M., Cowan, W. M. & Vagelos, P. R. Proc. natn. Acad. Sci. U.S.A. 71, 2183–2187 (1974).
Black, M. M. & Lasek, R. J. J. Cell Biol. 86, 616–623 (1980).
Bamburg, J. R., Bray, D. & Chapman, K. Nature 321, 788–790 (1986).
Nixon, R. A. & Logvinenko, K. B. J. Cell Biol. 102, 647–659 (1986).
Weisenberg, R. C. et al. Science 238, 1119–1122 (1987).
Kosik, K. S. & Finch, E. A. J. Neurosci. 7, 3142–3153 (1987).
Okabe, S. & Hirokawa, N. J. Cell biol. 107, 651–664 (1988).
Okabe, S. & Hirokawa, N. Proc. natn. Acad Sci. U.S.A. 86, 4127–4131 (1989).
Hollenbeck, P. J. J. Cell Biol. 108, 223–227 (1989).
Lim, S.-S., Sammak, P. J. & Borisy, G. G. J. Cell Biol. 109, 253–263 (1989).
Vigers, G. P. A., Coue, M. & Mclntosh, J. R. J. Cell Biol. 107, 1011–1024 (1988).
Keith, C. H. Science 235, 337–339 (1987).
Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E. L. & Webb, W. W. Biophys. J. 16, 1055–1069 (1976).
Wolf, D. E. Meth. Cell Biol. 30, 271–306 (1989).
Kreis, T. E., Geiger, B. & Schlessinger, J. Cell 29, 835–845 (1982).
Salmon, E. D., Saxton, W. M., Leslie, R. J., Karow, M. L. & Mclntosh, J. R. J. Cell Biol. 99, 2157–2164 (1984).
Oblinger, M. M., Brady, S. T., McQuarrie, I. G. & Lasek, R. J. J. Neurosci. 7, 453–462 (1987).
Tashiro, T. & Komiya, Y. J. Neurosci. 9, 760–768 (1989).
Sammak, P. J. & Borisy, G. G. Nature 332, 724–726 (1988).
Schulze, E. & Kirschner, M. Nature 334, 356–359 (1988).
Wang, Y. L. J. Cell Biol. 101, 597–602 (1985).
Okabe, S. & Hirokawa, N. J. Cell Biol. 109, 1581–1595 (1989).
Vikstrom, K. L., Borisy, G. G. & Goldman, R. D. Proc. natn. Acad. Sci. U.S.A. 86, 549–553 (1989).
Kellog, D. R., Mitchison, T. J. & Alberts, B. M. Development 103, 675–686 (1988).
Goldenberg, S. S. S. & De Boni, U. J. Neurobiol. 14, 195–206 (1983).
Hamaguchi, Y., Toriyama, M., Sakai, H. & Hiramoto, Y. Cell Struct. Funct. 12, 43–52 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okabe, S., Hirokawa, N. Turnover of fluorescently labelled tubulin and actin in the axon. Nature 343, 479–482 (1990). https://doi.org/10.1038/343479a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/343479a0
- Springer Nature Limited
This article is cited by
-
Mathematical models of neuronal growth
Biomechanics and Modeling in Mechanobiology (2022)
-
Axonal cytomechanics in neuronal development
Journal of Biosciences (2020)
-
Neurite elongation is highly correlated with bulk forward translocation of microtubules
Scientific Reports (2017)
-
Molecular mechanisms of dendrite stability
Nature Reviews Neuroscience (2013)
-
Rapid movement of axonal neurofilaments interrupted by prolonged pauses
Nature Cell Biology (2000)