Abstract
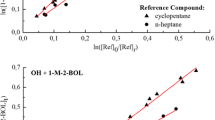
CONCERN has been expressed over the use of the halo-genated anaesthetics halothane (CF3CClBrH), enflurane (CF2HOCF2CFC1H) and isoflurane (CF2HOCHC1CF3) because of their potential for stratospheric ozone destruction1. Halogenated species also contribute to global warming2. The significance of the anaesthetics in stratospheric ozone loss or in 'greenhouse' heating depends on their atmospheric lifetimes. Because reaction with hydroxyl (OH) radicals is likely to be the main homogeneous sink for these species in the troposphere, we have measured absolute rates of reaction with OH. Comparison with a one-dimensional model3 indicates that the lifetimes of halothane, enflurane and isoflurane with respect to this reaction are 2, 6 and 5 years, respectively. Thus the small production of the anaesthetics is not offset by anomalously long atmospheric lifetimes to give a large atmospheric burden of the compounds. The anaesthetics will con-tribute at most a fraction of ∼5x 10-4 to the total atmospheric content of chlorine-containing species.
Similar content being viewed by others
References
Nørreslet, J. et al. Lancet i, 719 (1989).
WMO Global Ozone Research and Monitoring Project Atmospheric Ozone Report 16 (World Meteorological Organisation, Geneva, 1985).
Hammitt, J. K. et al. Nature 330, 711–716 (1987).
Goth, A. Medical Pharmacology (C. V. Cosby Co., St Louis, 1981).
Rogers, R. C. & Ross, J. A. S. Lancet i, 1209–1210 (1989).
Finlayson-Pitts, B. J. & Pitts, J. N. Jr Atmospheric Chemistry (Wiley Interscience, New York, 1986).
Dumas, J.-M. et al. Can. J. Spectrosc. 26, 102–108 (1981).
Lewis, B. R., Berzius, L. & Carver, J. M. Appl. Opt. 25, 2647–2648 (1986).
Burrows, J. P., Wallington, T. J. & Wayne R. P. JCS Faraday Trans. II 79, 111–122 (1983).
Boodaghians, R. B., Hall, I. W. & Wayne, R. P. JCS Faraday Trans. II 83, 529–538 (1987).
NASA Panel for Data Evaluation Chemical Kinetics and Photochemical Data for use in Stratospheric Modeling, No. 8 JPL Publn No. 87-41 (Jet Propulsion Laboratory. Pasadena, 1987).
Prinn, R. et al Science 238, 945–950 (1987).
Rogers, J. D. & Stephens, R. D. J. geophys. Res. 93, 2423–2428 (1988).
Liu, T. & Ji, L. Pharm. Ind. 4, 37–39 (1984).
CMA Fluorocarbon Program Panel Production, Sales and Calculated Release of CFC11 and CFC12 through 1986 (Chemical Manufacturers Association, Washington, DC, 1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, A., Canosa-Mas, C., Parr, A. et al. Tropospheric lifetimes of halogenated anaesthetics. Nature 341, 635–637 (1989). https://doi.org/10.1038/341635a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/341635a0
- Springer Nature Limited
This article is cited by
-
Reduction of greenhouse gases emission through the use of tiletamine and zolazepam
Scientific Reports (2022)
-
Chemical analysis of surgical smoke by infrared laser spectroscopy
Applied Physics B (2012)
-
Ultraviolet absorption spectra of some Br-containing haloalkanes
Journal of Atmospheric Chemistry (1995)
-
Rate constants for reactions of OH radicals with some Br-containing haloalkanes
Journal of Atmospheric Chemistry (1993)