Abstract
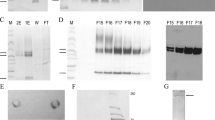
TRANSFORMING growth factor-β (TGF-β) (reviewed in refs 1–3) is a family of molecules that are made up as disulphide-bonded dimers of at least three different types of homologous polypeptides4–8. The active molecules are cleaved from the C termini of precursors4,6–8. TGF-β1, like other forms of TGF-β, is synthesized and secreted in a latent high relative molecular mass form (L-TGF-β1) from which active TGF-β1 can be released by transient and probably unphysiological acidification9–12. The latent complex from human platelets contains one dimeric TGF-β1 molecule, which is noncovalently associated with a disulphide-bonded complex of one dimeric remnant of the precursor and a single molecule of the so-called TGF-β1 binding protein (TGF-01-BP)11,13. We report here that enzymatic removal in vitro of the carbohydrate structures in the remnant of the TGF-β1 precursor produces biologically active TGF-β1 from the latent complex, suggesting that carbohydrate structures are of importance in rendering TGF-β1 inactive in the complex in vivo.
Similar content being viewed by others
References
Sporn, M. B., Roberts, A. B., Wakefield, L. M. & de Crombrugghe, B. J. Cell Biol. 105, 1039–1045 (1987).
Moses, H. L. et al. in Growth Factors and Transformation, Cancer Cells 3 (eds Feramisco, J., Ozanne, B. & Stiles, C.) 65–71 (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1985).
Massagué, J. Cell 48, 437–438 (1987).
Derynck, R. et al. Nature 316, 701–705 (1985).
Cheifez, S. et al. Cell 48, 409–415 (1987).
de Martin, R. et al. EMBO J. 6, 3673–3677 (1987).
ten Dijke, P., Hansen, P., Iwata, K. K., Pieler, C. & Foulkes, J. G. Proc. natn. Acad. Sci. U.S.A. 85, 4715–4719 (1988).
Derynck, R. et al. EMBO J. 7, 3737–3743 (1988).
Pircher, R., Lawrence, D. A. & Jullien, P. Cancer Res. 44, 5538–5543 (1984).
Pircher, R., Jullien, P. & Lawrence, D. A. Biochem. biophys. Res. Commun. 136, 30–37 (1986).
Miyazono, K., Hellman, U., Wernstedt, C. & Heldin, C.-H. J. biol Chem. 263, 6407–6415 (1988).
Lyons, R. M., Keski-Oja, J. & Moses, H. L. J. Cell Biol. 106, 1659–1665 (1988).
Wakefield, L. M., Smith, D. M., Flanders, K. C. & Sporn, M. B. J. biol. Chem. 263, 7646–7654 (1988).
Massagué, J. Meth. Enzym. 146, 174–195 (1987).
Massagué, J. & Like, B. J. biol. Chem. 260, 2636–2645 (1985).
Brunner, A. M., Gentry, L. E., Cooper, J. A. & Purchio, A. F. Molec. cell. Biol. 8, 2229–2232 (1988).
Elder, J. H. & Alexander, S. Proc. natn. Acad. Sci. U.S.A. 79, 4540–4544 (1982).
Thotakura, N. R. & Bahl, O. P. Meth. Enzym. 138, 350–359 (1987).
Purchio, A. F. et al. J. biol. Chem. 263, 14211–14215 (1988).
Hasilik, A. & Neufeld, E. F. J. biol. Chem. 255, 4946–4950 (1980).
Tabas, I. & Kornfeld, S. J. biol. Chem. 255, 6633–6639 (1980).
Shauer, R. Adv. Carbohyd. Chem. 40, 131–234 (1982).
Pilatte, Y., Bignon, J. & Lambré, C. R. Biochim. biophys. Acta 923, 150–155 (1987).
Morgan, D. O. et al. Nature 329, 301–307 (1987).
Frolik, C. A., Wakefield, L. M., Smith, D. M. & Sporn, M. B. J. biol. Chem. 259, 10995–11000 (1984).
Blobel, G. & Dobberstein, B. J. Cell Biol. 67, 835–851 (1975).
Morrissey, J. H. Analyt Biochem. 117, 307–310 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyazono, K., Heldin, CH. Role for carbohydrate structures inTGF-β1 latency. Nature 338, 158–160 (1989). https://doi.org/10.1038/338158a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/338158a0
- Springer Nature Limited
This article is cited by
-
Molecular forms of BMP15 and GDF9 in mammalian species that differ in litter size
Scientific Reports (2023)
-
Targeting TGF-β signal transduction for fibrosis and cancer therapy
Molecular Cancer (2022)
-
Role of glycosylation in TGF-β signaling and epithelial-to-mesenchymal transition in cancer
Protein & Cell (2021)
-
Choreographing Metastasis to the Tune of LTBP
Journal of Mammary Gland Biology and Neoplasia (2011)
-
Controversy surrounding the increased expression of TGFβ1 in asthma
Respiratory Research (2007)