Abstract
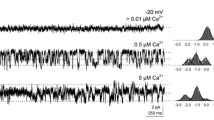
Olfactory transduction is thought to be initiated by the binding of odorants to specific receptor proteins in the cilia of olfactory receptor cells (reviewed in refs 1–3). The mechanism by which odorant binding could initiate membrane depolarization is unknown, but the recent discovery of an odorant-stimulated adenylate cyclase in purified olfactory cilia4,5 suggests that cyclic AMP may serve as an intracellular messenger for olfactory transduction. If so, then there might be a conductance in the ciliary plasma membrane which is controlled by cAMP. Here we report that excised patches of ciliary plasma membrane, obtained from dissociated receptor cells, contain a conductance which is gated directly by cAMP. This conductance resembles the cyclic GMP-gated conductance that mediates phototransduction in rod and cone outer segments6,7, but differs in that it is activated by both cAMP and cGMP. Our data provide a mechanistic basis by which an odorant-stimulated increase in cyclic nucleotide concentration could lead to an increase in membrane conductance and therefore, to membrane depolarization. These data suggest a remarkable similarity between the mechanisms of olfactory and visual transduction and indicate considerable conservation of sensory transduction mechanisms.
Similar content being viewed by others
References
Rhein, L. D. & Cagan, R. H. in Biochemistry of Taste and Olfaction (eds Cagan, R. H. & Kare, M. R.) 47–65 (Academic, New York, 1981).
Getchell, T. V. Physiol. Rev. 66, 772–818 (1986).
Lancet, D. A. Rev. Neurosci. 9, 329–355 (1986).
Pace, U., Hanski, E., Salomon, Y. & Lancet, D. Nature 316, 255–258 (1985).
Sklar, P. B., Anholt, R. H. & Snyder, S. H. J. biol. Chem. 261, 15538–15543 (1986).
Fesenko, E. E., Kolesnikov, S. S. & Lyubarsky, A. L. Nature 313, 310–313 (1985).
Haynes, L. W. & Yau, K.-W. Nature 317, 61–64 (1985).
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. Pflügers Arch. ges. Physiol. 391, 85–100 (1981).
Reese, T. S. J. Cell Biol. 25, 209–230 (1965).
Corey, D. P. & Stevens, C. F. in Single Channel Recording (eds Sakmann, B. & Neher, E.) 53–68 (Plenum, New York, 1983).
Sakmann, B. & Neher, E. in Single Channel Recording (eds Sakmann, B. & Neher, E.) 37–51 (Plenum, New York, 1983).
Matthews, G. Soc. Neurosci. Abstr. 11, 1130 (1985).
Yau, K.-W. & Haynes, L. W. Biophys. J. 49, 33a (1986).
Haynes, L. W., Kay, A. R. & Yau, K.-W. Nature 321, 66–70 (1986).
Zimmerman, A. L. & Baylor, D. A. Nature 321, 70–72 (1986).
Trotier, D. & MacLeod, P. Brain Res. 268, 225–237 (1983).
Suzuki, N. in Food Intake and Chemical Senses (eds Katsuki, Y., Sato, M., Takagi, S. F. & Oomura, Y.) 13–22 (University of Tokyo Press, 1977).
Anderson, P. A. V. & Hamilton, K. A. Neuroscience (in the press).
Getchell, T. V., Heck, G. L. & DeSimone, J. A. Biophys. J. 29, 397–412 (1980).
Getchell, T. V. & Shepherd, G. M. J. Physiol., Lond. 282, 541–560 (1978).
Masukawa, L. M., Hedlund, B. & Shepherd, G. M. J. Neurosci. 5, 136–141 (1985).
Takagi, S. F. in Handbook, of Sensory Physiology Vol. 4 (ed. Beidler, L. M.) 75–94 (Springer, New York, 1971).
Rhein, L. D. & Cagan, R. H. Proc. natn. Acad. Sci. U.S.A. 77, 4412–4416 (1980).
Adamek, G. D., Gesteland, R. C., Mair, R. G. & Oakley, B. Brain Res. 310, 87–97 (1984).
Huque, T. & Bruch, R. C. Biochem. biophys. Res. Commun. 137, 36–42 (1986).
Sklar, P. B., Anholt, R. R. H. & Snyder, S. H. J. Neurosci. Abstr. 12, 1178 (1986).
Vodyanoy, V. & Murphy, R. B. Science 220, 717–719 (1983).
Fesenko, E. E., Novoselov, V. I., Pervukin, G. Y. & Fesenko, N. K. Biochim. biophys. Acta 466, 347–356 (1977).
Vinnikov, J. A. Cold Spring Harb. Symp. quant. Biol. 30, 293–300 (1965).
Jencks, W. P. in Chemical Recognition in Biology (eds Chapeville, F. & Haenni, A.-L.) 3–25 (Springer, New York, 1980).
Graziadei, P. P. C. in Handbook of Sensory Physiology Vol. 4 (ed. Beidler, L. M.) 27–58 (Springer, New York, 1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakamura, T., Gold, G. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature 325, 442–444 (1987). https://doi.org/10.1038/325442a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/325442a0
- Springer Nature Limited
This article is cited by
-
GPRC5C regulates the composition of cilia in the olfactory system
BMC Biology (2023)
-
Conformational trajectory of allosteric gating of the human cone photoreceptor cyclic nucleotide-gated channel
Nature Communications (2023)
-
Contribution of TRPC3-mediated Ca2+ entry to taste transduction
Pflügers Archiv - European Journal of Physiology (2023)
-
Structure of the human cone photoreceptor cyclic nucleotide-gated channel
Nature Structural & Molecular Biology (2022)
-
Protein interaction, cytotoxic, transcriptomic and proteomic responses to structurally distinct EPAC1 activators in HUVECs
Scientific Reports (2022)