Abstract
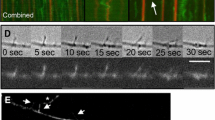
The actions of actin-based microfilaments in cell motility suggest a possible role in the mechanism of fast axonal transport1–3, but the pharmacological data evaluating their role in this process are equivocal4–6. Moreover, microfilaments are difficult to preserve and identify in ultrastructural studies7, so the organization and function of axonal actin has remained uncertain. We have now evaluated the role of actin microfilaments in intracellular transport of membranous organelles using video-enhanced contrast microscopy and gelsolin to analyse fast axonal transport directly in isolated axoplasm from the squid giant axon. With this preparation it is possible to perfuse axoplasm with large molecules that do not cross the plasmalemma, while controlling cation levels. The 90,000-molecular weight protein gelsolin depolymerizes actin microfilaments in micromolar Ca2+, but not in the absence of Ca2+. Axonal transport of membranous organelles has previously been shown to be unaffected by levels of Ca2+ up to 10 µM8. In the presence of EGTA, gelsolin has no effect on the movement of membranous organelles, but in the presence of 10 µM Ca2+ it completely blocks transport of all membranous organelles. No changes in the organization of the axoplasm were detected. These results and results using other probes for actin are consistent with the hypothesis that actin-based microfilaments are involved in the movement of membranous organelles in the axon.
Similar content being viewed by others
References
Bray, D. Biochimie 59, 1–6 (1977).
Goldberg, D. in Axoplasmic Transport (ed. Weiss, D.) 73–80 (Springer, Berlin, 1982).
Isenberg, G., Schubert, P. & Kreutzberg, G. in Axoplasmic Transport (ed. Weiss, D.) 314–321 (Springer, Berlin, 1982).
Isenberg, G., Schubert, P. & Kreutzberg, G. Brain Res. 194, 588–593 (1980).
Goldberg, D., Harris, D., Lubit, B. & Schwartz, J. Proc. natn. Acad. Sci. U.S.A. 77, 7448–7452 (1980).
Goldberg, D. Proc. natn. Acad. Sci. U.S.A. 79, 4818–4822 (1982).
Forer, A. Meth. Cell Biol. 25, 131–142 (1982).
Brady, S., Lasek, R. & Allen, R. Cell Motility (submitted).
Lazarides, E. & Lindberg, U. Proc. natn. Acad. Sci. U.S.A. 71, 4742–4746 (1974).
Davies, P., Bechtel, P. & Pastan, I. FEBS Lett. 77, 228–232 (1977).
Brown, S. & Spudich, J. J. Cell Biol. 88, 487–491 (1981).
McLean-Fletcher, S. & Pollard, T. Cell 20, 329–341 (1980).
Mojris, J. thesis, Case Western Reserve Univ. (1981).
Morris, J. & Lasek, R. J. Cell Biol. 92, 192–198 (1982).
Wehland, J., Osborn, M. & Weber, K. Proc. natn. Acad. Sci. U.S.A. 74, 5613–5617 (1977).
Brady, S. T., Morris, J. R. & Lasek, R. J. (in preparation).
Allen, R., Allen, N. & Travis, J. Cell Motility 1, 291–302 (1981).
Alien, R.D. & Allen, N. J. Microsc. 129, Pt 1, 3–17 (1983).
Brady, S., Lasek, R. & Alien, R. Science 218, 1129–1131 (1982).
Alien, R., Metuzals, J., Tasaki, I., Brady, S. & Gilbert, S. Science 218, 1127–1129 (1982).
Yin, H., Albrect, J. & Fattoum, A. J. Cell Biol. 91, 901–906 (1981).
Yin, H. L., Kwiatkowski, D., Mole, J. J. biol Chem. 259, 5271–5276 (1984).
Yin, H., Hartwig, J., Maruyama, K. & Stossel, T. J. biol. Chem. 256, 9693–9697 (1981).
Laemmli, U. Nature 227, 680–685 (1970).
O'Farrell, P. J. biol. Chem. 250, 4007–4021 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brady, S., Lasek, R., Allen, R. et al. Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature 310, 56–58 (1984). https://doi.org/10.1038/310056a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/310056a0
- Springer Nature Limited
This article is cited by
-
Synaptic vesicle traffic is supported by transient actin filaments and regulated by PKA and NO
Nature Communications (2020)
-
A Perspective on Neuronal Cell Death Signaling and Neurodegeneration
Molecular Neurobiology (2010)
-
Actin-dependent organelle movement in squid axoplasm
Nature (1992)
-
Molecular motors in axonal transport
Molecular Neurobiology (1992)
-
Posttranslational modifications of nerve cytoskeletal proteins in experimental diabetes
Molecular Neurobiology (1992)