Abstract
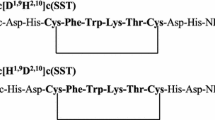
Conformational analysis has resulted in the design and synthesis of somatostatin analogues which show increased duration of action1–3. The introduction of covalent conformational constraints and elimination of amino acids that are not required led to the synthesis of the highly active bicyclic analogue I, cyclo(Aha-Cys-Phe-D-Trp-Lys-Thr-Cys)2,3, which showed potency equal to or greater than that of somatostatin for the inhibition of growth hormone release in vitro and in vivo, as well as for the inhibition of glucagon and insulin release in vivo (Aha, 7-aminoheptanoic acid). These results suggested that the amino acids -Phe-D-Trp-Lys-Thr- of this analogue and the corresponding residues 7–10 of somatostatin contain all the elements necessary for the expression of the above biological activities, and that the fragment -Cys-Aha-Ctys- serves as a conformational constraint, allowing the tetrapeptide to attain a bioactive conformation. It was concluded that such constraint also results in the observed reduced susceptibility to metabolism by peptidases such as trypsin and has permitted both a long duration of action and oral activity2,3. If the sole purpose of the -Cys-Aha-Cys- sequence is indeed only conformational constraint, then it should be possible to design alternative, simpler constraining moieties. We have used a computer modelling and graphics system4 to examine possible alternative molecular fragments and report here the synthesis of a highly active cyclic hexapeptide analogue of somatostatin.
Similar content being viewed by others
References
Veber, D. F. et al. Proc. natn. Acad. Sci. U.S.A. 75, 2636–2640 (1978).
Veber, D. F. in Peptides: Proc. 6th Am. Peptide Symp., (eds Gross, E. & Meienhofer, J.) 409–419 (Pierce Chemical Co., Rockford, Illinois, 1979).
Veber, D. F. et al. Nature 280, 512–514 (1979).
Gund, P., Andose, J. D., Rhodes, J. B. & Smith, G. M. Science 280, 1425–1431 (1980).
Arison, B. H., Hirschmann, R. & Veber, D. F. Bioorg. Chem. 7, 447–451 (1978).
Chandrasekaren, R., Lakshminarayanan, A. V., Pandya, U. V. & Ramachandran, G. N. Biochim. biophys. Acta 303, 14–27 (1973).
Brown, J. N. & Teller, R. G. J. Am. chem. Soc. 98, 7565–7569 (1976).
Hossain, M. B. & van der Helm, D. J. Am. chem. Soc. 100, 5191–5198 (1978).
Strachan, R. G. et al. J. med. Chem. 22, 586–588 (1979).
Gerich, J. E. Metabolism 27, 1283 (1978).
Veber, D. F. & Saperstein, R. A. Rep. med. Chem. 14, 209 (1979).
Finney, D. J. Statistical Methods in Biological Assay Ch. 4, 99–138 (Griffin, London, 1964).
Vale, W. & Grant, G. Meth. Enzym. 37, 5–93 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Veber, D., Freidinger, R., Perlow, D. et al. A potent cyclic hexapeptide analogue of somatostatin. Nature 292, 55–58 (1981). https://doi.org/10.1038/292055a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/292055a0
- Springer Nature Limited
This article is cited by
-
An amide to thioamide substitution improves the permeability and bioavailability of macrocyclic peptides
Nature Communications (2023)
-
Preparation of 155Tb-labeled short somatostatin analog
Russian Chemical Bulletin (2023)
-
Synthesis, in vitro biological activity and docking of new analogs of BIM-23052 containing unnatural amino acids
Amino Acids (2019)
-
Synthetic Peptide Analogs of Somatostatin: Trends in the Synthesis of and Prospects in the Search for New Anticancer Drugs
Pharmaceutical Chemistry Journal (2015)
-
Synthesis and in vitro antitumor activity of new octapeptide analogs of somatostatin containing unnatural amino acids
Amino Acids (2015)