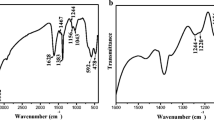
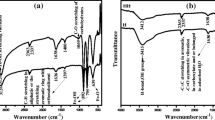
Abstract
THE adsorption of phosphate on aluminium and iron hydrous oxides is of practical importance in soils because of its influence on the availability of this nutrient to plants. One aspect of phosphate adsorption is the release of hydroxyl ions into the solution. This paper reports a quantitative relationship between divalent phosphate adsorbed on, and hydroxyl ions released from, hydrous alumina at different phosphate concentrations. From the relationship it is proposed that the phosphate ions are bonded to aluminium atoms forming both monodendate and bridged complexes.
Similar content being viewed by others
References
Rajan, S. S. S., Perrott, K. W., and Saunders, W. H. M., J. Soil Sci., (in the press),
Parks, G. A., and deBruyn, P. L., J. phys. Chem., 66, 967–973 (1962).
Rajan, S. S. S., N.Z. Jl Sci. (in the press).
Hingston, A. J., Atkinson, R. J., Posner, A. M., and Quirk, J. P., Nature, 215, 1459–1461 (1967).
Kafkafi, U., Posner, A. M., and Quirk, J. P., Soil Sci. Soc. Am. Proc., 31, 348–353 (1967).
Atkinson, R. J., Posner, A. M., and Quirk, J. P., J. inorg. nucl. Chem., 34, 2201–2211 (1972).
Hingston, F. J., Posner, A. M., and Quirk, J. P., J. Soil Sci., 25, 16–26 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RAJAN, S. Adsorption of divalent phosphate on hydrous aluminium oxide. Nature 253, 434–436 (1975). https://doi.org/10.1038/253434a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/253434a0
- Springer Nature Limited
This article is cited by
-
Effect of Long-Term Effluent Recharge on Phosphate Sorption by Soils in a Wastewater Reclamation Plant
Water, Air, and Soil Pollution (2005)
-
The chemistry of aquatic phosphate: inorganic processes in rivers
Hydrobiologia (1993)
-
Phosphate adsorption on goethite (α-FeOOOH)
Nature (1976)
-
Changes in net surface charge of hydrous alumina with phosphate adsorption
Nature (1976)
-
Charge relationships of phosphate sorption
Nature (1975)