Abstract
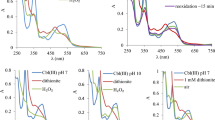
COBALT complexes of dimethylglyoxime (CH3C:NOH.C:NOH.CH3), commonly known as cobaloximes, form alkyl derivatives with unusually stable cobalt–carbon bonds1,2. These are regarded by some workers as ‘model’ compounds for vitamin B12, a biological alkylating agent, the action of which depends on the reversible formation of a similar Co–C bond3–5. Various mechanisms have been postulated for this reaction6. The mechanisms of transalkylation reactions undergone by cobaloximes are thus of great interest; such reactions have been investigated with Hg2+ (refs 7 and 8) and with Cr2+ (ref. 9). For comparison we are studying the reaction kinetics of other cobaloximes with Cr2+. These are apparently electron–transfer reactions10–12, and should also be considered in the context of the large volume of work that has been done in that field.
Similar content being viewed by others
References
Dodd, D., and Johnson, M. D., J. organometal. Chem., 52, 1 (1973).
Schrauzer, G. N., Acc. Chem. Res., 1, 97 (1968).
Pratt, J. M., The Inorganic Chemistry of Vitamin B12 (Academic Press, New York, (1972).
Prince, R. H., and Stotter, D. A., J. inorg. nucl. Chem., 35, 321 (1973).
Brown, D. G., Prog. inorg. Chem., 18, 177 (1973).
Agnes, G., et al., Biochim. biophys. Acta, 252, 207 (1971).
Adin, A., and Espenson, J. H., J. Chem. Soc., Chem. Commun., 653 (1971).
Schrauzer, G. N., Tetrahedron Lett., 275 (1971).
Espenson, J. H., and Schveima, J. S., J. Am. chem. Soc., 95, 4468 (1973).
Taube, H., and Gould, E. S., Acc. chem. Res., 2, 321 (1969).
Sykes, A. G., Adv. Chem. Radiochem., 10, 153 (1967).
Linck, R. G., Reaction Mechanisms in Inorganic Chemistry, 9, 303 (1972).
Gillard, R. D., and Wilkinson, G., J. chem. Soc., 6041 (1963).
Crumbliss, A. L., et al., J. chem. Soc., chem. Commun., 415 (1973).
Endicott, J. F., et al., J. Am. chem. Soc., 95, 5097 (1973).
Maki, N., Nature, 188, 227 (1960).
Schrauzer, G. N., and Windgassen, R. J., J. Am. chem. Soc., 88, 3738 (1966).
Hohokabe, Y., and Yamazaki, N., Bull. chem. Soc. Japan, 44, 1563 (1971).
Schrauzer, G. N., and Windgassen, R. J., J. Am. chem. Soc., 89, 1999 (1967).
Kolthoff, I. M., and Lingane, J. J., Polarography (Interscience, New York, 1941).
Latimer, W. M., The Oxidation States of the Elements and their Potentials in Aqueous Solution (Prentice-Hall, New York, 1938).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PRINCE, R., SEGAL, M. Cr2+-cobaloxime electron transfer reactions. Nature 249, 246–247 (1974). https://doi.org/10.1038/249246a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/249246a0
- Springer Nature Limited