Abstract
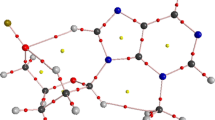
IN a nucleoside the rotation of the nucleobase about the glycosidic bond relative to the sugar moiety is sterically hindered and two conformational ranges, syn and anti, are preferred1. Theoretical2–5 and spectroscopic6–8 investigations suggest that the anti conformation is slightly more energetically favoured than the syn. In the crystalline state, this is also true for purine nucleosides but all pyrimidine nucleosides, except 4-thiouridine9 (a tRNA minor constituent), crystallize in anti conformation. 4-Thiouridine may occur in syn conformation because of the stabilization by the peculiar hydrogen bonding and packing scheme or the particular C(3′)-endo-C(4′)-exo puckering* of the ribose unit. The correlation between conformation about the glycosidic bond and the sugar puckering in nucleosides was further studied by X-ray analysis of 6-methyluridine (Fig. 1). This species, according to nuclear magnetic resonance studies6, exists in the syn conformation in aqueous solution because of the bulky methyl group in position 6 of the pyrimidine ring.
Similar content being viewed by others
References
Donohue, J., and Trueblood, K. N., J. Mol. Biol., 2, 363 (1960).
Haschemeyer, A. E. V., and Rich, A., J. Mol. Biol., 27, 369 (1967).
Lakshminarayanan, A. V., and Sasisekharan, V., Biochim. Biophys. Acta, 204, 49 (1970).
Berthod, H., and Pullman, B., Biochim. Biophys. Acta, 232, 595 (1971).
Kang, S., J. Mol. Biol., 58, 297 (1971).
Schweizer, M. P., Witkowski, J. T., and Robins, R. K., J. Amer. Chem. Soc., 93, 277 (1971).
Hart, P. A., and Davis, J. P., J. Amer. Chem. Soc., 91, 512 (1969).
Rogers, G. T., and Ulbricht, T. L. V., Biochem. Biophys. Res. Commun., 39, 419 (1970).
Saenger, W., and Scheit, K. H., J. Mol. Biol., 50, 153 (1970).
Karle, J., and Hautpman, H., Acta Cryst., 9, 635 (1956).
Germain, G., Main, P., and Woolfson, M. M., Acta Cryst., B 26, 274 (1970) and A 27, 368 (1971).
Saenger, W., and Eckstein, F., J. Amer. Chem. Soc., 92, 4712 (1970).
Rao, S. T., and Sundaralingam, M., J. Amer. Chem. Soc., 92, 4963 (1970).
Saenger, W., J. Amer. Chem. Soc., 93, 3035 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SUCK, D., SAENGER, W. & VORBRÜGGEN, H. Conformation of 6-Methyluridine — a Pyrimidine Nucleoside in the syn Conformation. Nature 235, 333–334 (1972). https://doi.org/10.1038/235333a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/235333a0
- Springer Nature Limited
This article is cited by
-
Structures of isopropylidene nucleoside derivatives: implications for ribose ring flexibility under external cyclic constraints
Proceedings / Indian Academy of Sciences (1984)
-
Left-handed DNA helices
Nature (1980)