Abstract
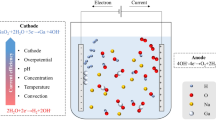
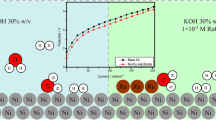
As a means of production, electrolysis of gallium from only alkaline and not acidic solutions has been reported1. The principal difficulties reported using acidic solutions are: (a) poor current efficiencies; (b) formation of solid gallium oxychloride2.
Similar content being viewed by others
References
Hampel, C. A., Rare Metals Handbook, sec. ed., 185 (Reinhold Publishing Corp., New York, 1961).
Papp, E., and Solymar, K., Acta Chim. Hung., 24, 451 (1960).
Hutter, J. C., and Peyron, A., C.R. Congr. intern. Chim. Ind., 31e, Liège, 1958 (publ. as Indust. Chim. belge, Supp.), 1, 801 (1959).
Saltman, W. M., and Nachtrieb, N. H., J. Electrochem. Soc., 100, 126 (1953).
Information on Gallium (Inform. Bull)., Aluminium-Industrie-Aktien-Gesellschaft, Neuhausen am Rheinfall/Switzerland (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
STEARNS, R. Production of Gallium by Continuous Electrodeposition from Acidic Solutions. Nature 203, 749–750 (1964). https://doi.org/10.1038/203749b0
Published:
Issue Date:
DOI: https://doi.org/10.1038/203749b0
- Springer Nature Limited