Abstract
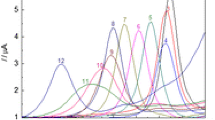
THE investigation of the mechanism of electro-oxidation is hindered by the lack of information about the reaction products and intermediates. These can be determined in some cases by ordinary analytical methods. In some cases a current-voltage curve obtained by application of a voltage sweep to the electrode during interruptions of the electro-oxidation can be used for determination of products of the electrode reaction, but usually does not give unambiguous results.
Similar content being viewed by others
References
Giner, J., Electrochem. Acta, 9, 63 (1964).
Němec, L. (in the press).
Valenta, P., Coll. Czech. Chem. Comm., 25, 853 (1960).
Levich, V. G., Physicochemical Hydrodynamics (Prentice-Hall, New Jersey, 1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
VALENTA, P., KORYTA, J. Determination of Electro-oxidation Products using an Auxiliary Mercury Electrode. Nature 203, 639–640 (1964). https://doi.org/10.1038/203639a0
Issue Date:
DOI: https://doi.org/10.1038/203639a0
- Springer Nature Limited