Abstract
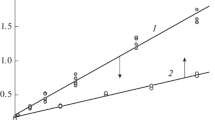
WHEN a dilute solution of 1,3,5-trinitrobenzene in ethanol is treated with sodium ethoxide, a yellow solution is formed. A similar colour is produced when sodium ethoxide is added to 2,4,6-trinitroanisole. The rates of development of the colours have been studied by Ainscough and Caldin1, who found that there are two colour-producing reactions: (i) a ‘fast’ reaction the product of which they suggest is a charge-transfer complex, and (ii) a ‘slow’ reaction, which probably produces an addition compound where OEt is covalently bonded to the nucleus, as was suggested by Meisenheimer2 for the solid obtained in this reaction (I, R = OCH3). With 1,3,5,-trinitrobenzene and sodium ethoxide only one reaction was observed, and on the basis of similar rates of reaction and similar effects of acid on the two systems, Ainscough and Caldin suggested that the product is a charge-transfer complex analogous to the ‘fast’ reaction with 2,4,6-trinitroanisole. The absorption spectra of the trini-trobenzene + sodium ethoxide obtained by them did not resemble that of trinitroanisole + sodium ethoxide.
Similar content being viewed by others
References
Ainscough, J. B., and Caldin, E. F., J. Chem. Soc., 2528 (1956).
Meisenheimer, J., Ann. der Chemie, 323, 205 (1902).
Foster, R., Nature, 176, 746 (1955).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FOSTER, R. Interaction of 1,3,5-Trinitrobenzene with Ethoxide Ion. Nature 183, 1042–1043 (1959). https://doi.org/10.1038/1831042b0
Issue Date:
DOI: https://doi.org/10.1038/1831042b0
- Springer Nature Limited