Abstract
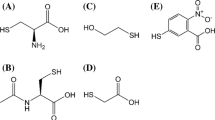
SINCE 1-fluoro-2,4-dinitrobenzene has fungicidal properties1 and is useful for the end-group and sequential analysis of proteins, its reaction with cysteine was investigated as part of a study of the mechanism of action of toxicants which function as alkylating agents. In the presence of ten molecular equivalents of cysteine the reaction was first order with respect to the disappearance of 1-fluoro-2,4-dinitrobenzene in dilute aqueous buffer. At pH 7.0 and 29° C. the half-time for the reaction was about 38 min. at an initial concentration of 2.7 × 10−5 mole of 1-fluoro-2,4-dinitrobenzene per litre. When the reaction was followed by measuring the formation of the final product by absorption readings at 360 mµ first-order kinetics were not strictly obeyed and the half-time was about 40 per cent longer. This suggested the presence of a transient intermediate.
Similar content being viewed by others
References
Miller, Harold J., Phytopath., 42, 470 (1952).
Burchfield, H. P., and Storrs, Eleanor E., Contrib. Boyce Thompson Inst. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BURCHFIELD, H. Molecular Rearrangement in the Reaction of Cysteine with 1-Fluoro-2,4-Dinitrobenzene. Nature 181, 49–50 (1958). https://doi.org/10.1038/181049b0
Issue Date:
DOI: https://doi.org/10.1038/181049b0
- Springer Nature Limited
This article is cited by
-
Transaldolase: A model for studies of isoenzymes and half-site enzymes
Molecular and Cellular Biochemistry (1973)
-
Instability of some of the Products Formed by the Action of N-methyl-N-nitrosourethane on Cysteine in vitro. Role of Neighbouring Groups in Enzymatic and Carcinogenic Action
Nature (1966)