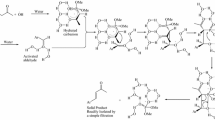
Abstract
THE alkylation of tertiary amines by neutral phosphate esters has been reported1, and in one instance self-methylation has been observed2. Thus, Andrews et al. found that (m-dimethyl phosphato)-N-dimethylaniline forms the quaternary zwitterion slowly at room temperature and rapidly on heating. Hobbiger3 found that the anticholinesterase activity of the dimethyl, diethyl and di-isopropyl ternary compounds increased in water, which suggests that (dialkyl phosphato)-N-dimethylalkylanilinium compounds are formed by the bimolecular reaction:  The dialkyl quaternary compounds are powerful inhibitors of cholinesterase4, whereas it is unlikely that the zwitterion is.
The dialkyl quaternary compounds are powerful inhibitors of cholinesterase4, whereas it is unlikely that the zwitterion is.
Similar content being viewed by others
References
Baddiley, J., Clark, V. M., Michalski, J. J., and Todd, A. R., J. Chem. Soc., 815 (1949).
Andrews, K. J. M., Atherton, F. R., Bergel, F., and Morrison, A. L., J. Chem. Soc., 780 (1952).
Hobbiger, F., Ph.D. thesis, University of London (1952).
Burgen, A. S. V., and Hobbiger, F., Brit. J. Pharm., 6, 593 (1951).
Aldridge, W. N., and Davison, A. N., Biochem. J., 55, 763 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HEATH, D. Self-Alkylation of some Dialkyl Phosphate Esters. Nature 179, 377–378 (1957). https://doi.org/10.1038/179377a0
Issue Date:
DOI: https://doi.org/10.1038/179377a0
- Springer Nature Limited
This article is cited by
-
�ber Vorkommen von Sulfoniumverbindungen in Metasystox (i) und Metasystox R und ihre physiologische Wirkung
F�hner-Wielands Sammlung von Vergiftungsf�llen Archiv f�r Toxikologie (1963)