Abstract
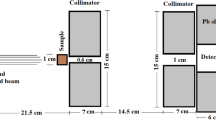
THE absolute yield of ferric ions produced on irradiation with cobalt-60 γ-rays and fast electrons of aerated solutions, 1–10 mM in ferrous ion and 0.1–2N in sulphuric acid, has been measured by various methods and a ‘G-value’ of 15.6 ± 0.4 ferric ions per 100 eV. absorbed is now well established1. Less information is available on the yield of this system with X-rays of 250 keV. and lower energy, which are commonly used in studies in radiation chemistry. Ionization methods of measurement of the energy absorbed from such radiation have limited accuracy, owing to the difficulty of correcting for the photoelectric absorption in the sulphur atoms present, to uncertainties in the parameters used in computing dose, and because of geometrical considerations. To minimize photoelectric absorption, it is obvious that the sulphuric acid content of the system should be as low as possible ; but the use of solutions less than 0.1 N in sulphuric acid is not recommended, this being the lower limit of the range of independence of yield on acid concentration2.
Similar content being viewed by others
References
Miller, “Actions Chimiques et Biologiques des Radiations”, 2me. série (Masson et Cie, Paris, 1956).
Dewhurst, Trans. Farad. Soc., 49, 1174 (1953).
Schwarz, J. Amer. Chem. Soc., 77, 4960 (1955).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BACK, M., MILLER, N. Use of Ferrous Sulphate Solutions for X-Ray Dosimetry. Nature 179, 321–322 (1957). https://doi.org/10.1038/179321a0
Issue Date:
DOI: https://doi.org/10.1038/179321a0
- Springer Nature Limited