Abstract
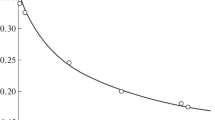
IN view of the importance which is attached to the photochemical oxidation of water in the understanding of the process of photosynthesis1, we would like to submit a scheme, which, as we show, agrees well with the data reported in the literature and our own experience in this field of research. Weiss and Porret2 were the first to investigate the photo-oxidation by ceric ions (a qualitative observation to this effect had been made by Baur3 in 1908). A more detailed study was carried out by Heidt and Smith4. The latter assumed the formation of an active ceric dimer being deactivated by cerous ions to account for their otherwise inexplicable observations as to dependence of the quantum yield on the concentration of ceric and cerous ions in solution. Spectrophotometric measurements carried out by us (to be published later) show conclusively that there is no dimer formation, and that in ceric perchlorate solutions in normal perchloric acid approximately 92 per cent of the ceric ion is present as the ion-pair complex Ce4+OH−. On the other hand, Heidt and Smith's kinetic results can be accounted for qualitatively and quantitatively on the basis of the following reaction scheme, which makes it also possible to explain in quantitative terms why in the case of ferric ions the quantum yield should be much smaller, and therefore under normal laboratory conditions scarcely measurable; as in our work on photo-initiated polymerization5,6 and oxidation7, we consider the primary step as an electron-transfer excitation with the ion-pair complex as the active species. This is the scheme we suggest:  From stationary-state kinetics we obtain:
From stationary-state kinetics we obtain: 
Similar content being viewed by others
References
Rabinowitch, “Photosynthesis”, 1, 69 (New York and London: Interscience Publishers, 1945).
Weiss and Porret, Nature, 139, 1019 (1937).
Baur, Z. phys. Chem., 63, 683 (1908).
Heidt and Smith, J. Amer. Chem. Soc., 70, 2476 (1948).
Evans and Uri, Nature, 164, 404 (1949).
Evans and Uri, J. Soc. Dyers and Colour., 65, 709 (1949).
Bates, Evans and Uri (in the course of publication).
Evans and Uri, Trans. Farad. Soc., 45, 224 (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
EVANS, M., URI, N. Photo-oxidation of Water by Ceric Ions. Nature 166, 602–603 (1950). https://doi.org/10.1038/166602b0
Issue Date:
DOI: https://doi.org/10.1038/166602b0
- Springer Nature Limited
This article is cited by
-
Cerium(IV)-driven oxidation of water catalyzed by mononuclear ruthenium complexes
Research on Chemical Intermediates (2014)
-
Role of hydrogen peroxide in the radiation induced reduction of mercuric chloride
Die Naturwissenschaften (1963)