Abstract
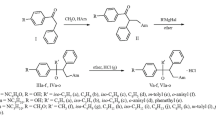
THIS compound (I) was prepared for the following reason: pyridine-3-sulphonic acid and its amide were found to inhibit bacterial growth, apparently by interference with nicotinic acid metabolism1. Sulphanilamide appears to act as a therapeutic agent by interfering with the utilization of p-aminobenzoic acid by the invading organisms2, but the 2-aminopyridine derivative(M. & B. 693) is in many cases more effective. Following a rule of thumb method common in chemotherapeutic research, the M. & B. 693 analogue of pyridine-3-sulphonic acid, (I), was prepared and its inhibitory powers compared with those of the parent acid and amide by in vitro tests analogous to those previously described1,2. The compound was found less active than the simple acid and amide.
Similar content being viewed by others
References
McIlwain, H., Brit. J. Exp. Path., 21, 136 (1940).
Woods, D. D., Brit. J. Exp. Path., 21, 74 (1940).
Fildes, P., Lancet, i, 955 (1940).
Walker, J., J. Chem. Soc., 686 (1940).
Gray, W. H., J. Chem. Soc., 1202 (1939).
Buttle, G. A. H., Gray, W. H., and Stephenson, D., Lancet, i, 1286 (1936).
Clemo, G. R., McIlwain, H., and Morgan, W. McG., J. Chem. Soc., 610 (1936).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MCILWAIN, H. Pyridine-3-sulphon-(2-pyridyl)-amide; a Note on the Modelling of Chemotherapeutic Agents. Nature 146, 653–654 (1940). https://doi.org/10.1038/146653a0
Published:
Issue Date:
DOI: https://doi.org/10.1038/146653a0
- Springer Nature Limited
This article is cited by
-
Dérivés simples de l'acide p-aminobenzoïque agissant comme sulfonamides et antisulfonamides
Antonie van Leeuwenhoek (1946)