Abstract
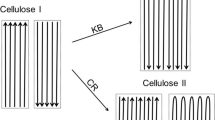
IF cellulose is precipitated from solution or liberated from its compounds with sodium hydroxide, the space lattice (a = 8·14 A., b = 10·3 A., c = 9·14 A., β= 62°) differs from that of native cellulose (a = 8·35 A., b = 10·3 A., c = 7·9 A., (β= 84°). In this form it is known as hydrate cellulose, although it is in fact of the same analytical composition as the native form, and is free from water. Hitherto it has not been possible to convert one modification directly into the other ; it was possible to pass from native cellulose via alkali cellulose to hydrate cellulose, and from this (as Barry, Peterson and King1 showed recently) by way of the ammonia compound back to native cellulose. Moreover, Hess and Gundermann2 have described the appearance of native cellulose along with hydrate cellulose during the washing out of alkali cellulose at 100° C. We find, however, that it is possible to convert hydrate cellulose directly into native cellulose.
Similar content being viewed by others
References
J. Amer. Chem. Soc., 58, 333 (1936).
Ber. deutsch. chem. Ges., 70, 527 (1937); see also Hess and collaborators in Z. phys. Chem., B, 7, 7 (1930).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MEYER, K., BADENHUIZEN, N. Transformation of Hydrate Cellulose into Native Cellulose. Nature 140, 281–282 (1937). https://doi.org/10.1038/140281b0
Issue Date:
DOI: https://doi.org/10.1038/140281b0
- Springer Nature Limited