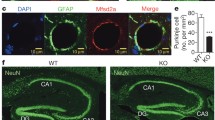
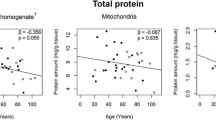
Abstract
Until recently, brain phospholipid metabolism was thought to consume only 2% of the ATP consumed by the mammalian brain as a whole. In this paper, however, we calculate that 1.4% of total brain ATP consumption is consumed for the de novo synthesis of ether phospholipids and that another 5% is allocated to the phosphatidylinositide cycle. When added to previous estimates that fatty acid recycling within brain phospholipids and maintenance of membrane lipid asymmetries of acidic phospholipids consume, respectively, 5% and 8% of net brain ATP consumption, it appears that phospholipid metabolism can consume up to 20% of net brain ATP consumption. This new estimate is consistent with recent evidence that phospholipids actively participate in brain signaling and membrane remodeling, among other processes.
Similar content being viewed by others
REFERENCES
Vierbuchen, M., Gunawan, J., and Debuch, H. 1979. Studies on the hydrolysis of 1–alkyl-sn-glycero-3–phosphoethanolamine in subcellular fractions of rat brain. Hoppe-Zeyler' Z. Physiol. Chem. 360:1091–1097.
Porcellati, G., Goracci, G., and Arienti, G. 1983. Lipid turnover. Pages 277–294, in Lajtha, A. (ed.), Handbook of neurochemistry, vol. 5. Plenum, New York.
Clarke, D. D. and Sokoloff, L. 1994. Circulation and energy metabolism of the brain. Pages 645–680, in Siegel, G. J., Agranoff, B. W., Albers, R. W., and Molinoff, M. D. (eds.), Basic neurochemistry: Molecular, cellular, and medical aspects. Raven Press, New York.
Ansell, G. B. 1973. Phospholipids in the nervous system. Pages 377–422, in Ansell, G. B., Hawthorne, J. N., and Dawson, R. M. C. (eds.), Form and function of phospholipids. Elsevier, New York.
Miller, S. L., Benjamins, J. A., and Morell, P. 1977. Metabolism of glycerophospholipids of myelin and microsomes in rat brain. J. Biol. Chem. 252:4025–4037.
Dawson, R. M. C. 1985. Enzymic pathways of phospholipid metabolism in the nervous system. Pages 45–78, in Eichberg, J. (ed.), Phospholipids in nervous tissues. John Wiley and Sons, New York.
Fisher, S. K. and Agranoff, B. W. 1985. The biochemical basis and functional significance of enhanced phosphatidate and phosphoinositide turnover. Pages 241–295, in Eichberg, J. (ed.), Phospholipids in nervous tissues. John Wiley and Sons, New York.
Horrocks, L. 1985. Metabolism and function of fatty acids in brain. Pages 173–199, in Eichberg, J. (ed.), Phospholipids in nervous tissues. John Wiley and Sons, New York.
Bazán, N. G. 1990. Supply of n-3 polyunsaturated fatty acids and their significance in the central nervous system. Pages 1–24, in Wurtman, R. J. and Wurtman, J. J. (eds.), Nutrition and the brain, vol. 8. Raven Press, New York.
Glaser, P. E. and Gross, R. W. 1994. Plasmenylethanolamine facilitates rapid membrane fusions: A stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry 33:5805–5812.
Purdon A. D. and Rapoport, S. I. 1998. Energy requirements for two aspects of phospholipid metabolism in mammalian brain. Biochem. J. 335:313–318.
Zachowski, A. and Gaudry-Talarmain, Y. M. 1990. Phospholipid transverse diffusion in synaptosomes: Evidence for the involvement of the aminophospholipid translocase. J. Neurochem. 55:1352–1356.
Williamson, P. and Schlegel, R. A. 1994. Back and forth: The regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol. Membr. Biol. 11:199–216.
Blank, M. L., Cress, E. A., Robinson, M., and Snyder, F. 1985. Metabolism of unique diarachidonoyl and linoleoylarachidonoyl species of ethanolamine and choline phosphoglycerides in rat testes. Biochim. Biophys. Acta 833:366–371.
Fisher, S. K., Heacock, A. M., and Agranoff, B. W. 1992. Inositol lipids and signal transduction in the nervous system: An update. J Neurochem 58:18–38.
Izumi, T. and Shimizu, T. 1995. Platelet-activating factor receptor: Gene expression and signal transduction. Biochim. Biophys. Acta 1259:317–333.
Cooper, J. R., Bloom, F. E., and Roth, R. H. 1996. The biochemical basis of neuropharmacology, 7th ed., Oxford University Press, Oxford.
Shetty, H. U., Smith, Q. R., Washizaki, K., Rapoport, S. I., and Purdon, A. D. 1996. Identification of two molecular species of rat brain phosphatidylcholine that rapidly incorporate and turn over arachidonic acid in vivo. J. Neurochem. 67:1702–1710.
Kuwae, T., Schmid, P. C., and Schmid, H. H. 1997. Alterations of fatty acyl turnover in macrophage glycerolipids induced by stimulation. Evidence for enhanced recycling of arachidonic acid. Biochim. Biophys. Acta 1344:74–86.
Zoeller, R. A., Lake, A. C., Nagan, N., Gaposchkin, D. P., Legner, M. A., and Lieberthal, W. 1999. Plasmalogens as endogenous antioxidants: Somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 338:769–776.
Chikhale, E. G., Balbo, A., Galdzicki, Z., Rapoport, S. I., and Shetty, H. U. 2001. Measurement of myo-inositol turnover in phosphatidylinositol: Description of a model and mass spectrometric method for cultured cortical neurons. Biochemistry 40:11114–11120.
Rosenberger, T. A., Oki, J., Purdon, A. D., Rapoport, S. I., and Murphy, E. J. 2002. Rapid synthesis and turnover of brain microsomal ether phospholipids in the adult rat. J. Lipid Res. 43:59–68.
Rana, R. S. and Hokin, L. E. 1990. Role of phosphoinositides in transmembrane signaling. Physiol. Rev. 70:115–64.
Hokin, L. E. and Dixon, J. F. 1993. The phosphoinositide signaling system. I. Historical background. II. Effects of lithium on the accumulation of second messenger inositol 1,4,5–trisphosphate in brain cortex slices. Prog. Brain Res. 98:309–315.
Agranoff, B. W. and Fisher, S. K. 1994. Phosphoinositides. Pages 417–448 in Siegel, G. J., Agranoff, B. W., Albers, R. W., and Molinoff, P. B. (eds.), Basic neurochemistry, 5th ed. Raven Press, New York.
Hajra, A. K. 1995. Glycerolipid biosynthesis in peroxisomes (microbodies). Prog. Lipid Res. 34:343–364.
Verhoeven, A. J., Tysnes, O. B., Aarbakke, G. M., Cook, C. A., and Holmsen, H. 1987. Turnover of the phosphomonoester groups of polyphosphoinositol lipids in unstimulated human platelets. Eur. J. Biochem. 166:3–9.
Bell, R. M. and Coleman, R. A. 1980. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 49:459–487.
Pollock, R. J., Hajra, A. K., and Agranoff, B. W. 1976. Incorporation of D-[3–3H, U-14C] glucose into glycerolipid via acyl dihydroxyacetone phosphate untransformed and viral-transformed BHK-21–c13 fibroblasts. J. Biol. Chem. 251:5149–5154.
Lee, T. C. 1998. Biosynthesis and possible biological functions of plasmalogens. Biochim. Biophys. Acta 1394:129–145.
Ford, D. A. and Gross, R. W. 1994. The discordant rates of sn-1 aliphatic chain and polar head group incorporation into plasmalogen molecular species demonstrate the fundamental importance of polar head group remodeling in plamalogen metabolism in rabbit myocardium. Biochemistry 33:1216–1222.
Hinkle, P. C., Kumar, M. A., Resetar, A., and Harris, D. L. 1991. Mechanistic stoichiometry of mitochondrial oxidative phosphorylation. Biochemistry 30:3576–3582.
Rolfe, D. F. S. and Brown, G. C. 1997. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77:731–747.
Sokoloff, L., Reivich, M., Kennedy, C., Des Rosiers, M. H., Patlak, C. S., Pettigrew, K. D., Sakurada, O., and Shinohara, M. 1977. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28:897–916.
Dobson, G. P. and Headrick, J. P. 1995. Bioenergetic scaling: Metabolic design and body-size constraints in mammals. Proc. Natl. Acad. Sci. USA 92:7317–7321.
Paltauf, F. 1994. Ether lipids in biomembranes. Chem. Phys. Lipids 74:101–139.
Snyder, F. 1996. Ether-linked lipids and their bioactive species: Occurrence, chemistry, metabolism, regulation, and function. Pages 183–210 in Vance, D. E. and Vance, J. E. (eds.), Biochemistry of lipids, lipoproteins, and membranes. Elsevier, New York.
Pediconi, M. F. and Barrantes, F. J. 1990. Brain asymmetry in phospholipid polar head group metabolism: Parallel in vivo and in vitro studies. Neurochem. Res. 15:25–32.
Tong, W. and Sun, G. Y. 1996. Effects of ethanol on phosphorylation of lipids in rat synaptic plasma membranes. Alcohol Clin. Exp. Res. 20:1335–1339.
Soukup, J. F., Friedel, R. O., and Shanberg, S. M. 1978. Microwave irradiation fixation for studies of polyphosphoinositide metabolism in brain. J. Neurochem. 30:635–637.
Nishihara, M. and Keenan, R. W. 1985. Inositol phospholipid levels of rat forebrain obtained by freeze-blowing method. Biochim. Biophys. Acta 835:415–418.
Reddy, T. S. and Bazan, N. G. 1987. Arachidonic acid, stearic acid, and diacylglycerol accumulation correlates with the loss of phosphatidylinositol 4,5–bisphosphate in cerebrum 2 seconds after electroconvulsive shock: Complete reversion of changes 5 minutes after stimulation. J. Neurosci. Res. 18:449–455.
Masuzawa, Y., Sugiura, T., Ishima, Y., and Waku, K. 1984. Turnover rates of the molecular species of ethanolamine plasmalogen of rat brain. J. Neurochem. 42:961–968.
Tabata, H., Bell, J. M., Miller, J. C., and Rapoport, S. I. 1986. Incorporation of plasma palmitate into the brain of the rat during development. Dev. Brain Res. 29:1–8.
Tysnes, O. B., Verhoeven, A. J., and Holmsen, H. 1988. Rates of production and consumption of phosphatidic acid upon thrombin stimulation of human platelets. Eur. J. Biochem. 174: 75–79.
Brusa, R., Eva, C., Oberto, A., Peila, R., Ricci Galamero, S., and Genazzani, E. 1992. Down regulation of muscarinic receptor subtypes messenger RNAs in rat primary culture of corticostriatal neurons. Pharmacol. Res. 25 (Suppl 1):121–122.
Hertz, L. and Peng, L. 1992. Energy metabolism at the cellular level of the CNS. Can J. Physiol. Pharmacol. 70 (Suppl):S145–S157.
Murphy, T. H., Wright, D. D., and Baraban, J. M. 1992. Phosphoinositide turnover associated with synaptic transmission. J. Neurochem. 59:2336–2339.
Rowlands, D. K., Kao, C., and Wise, H. 2001. Regulation of prostacyclin and prostaglandin E(2) mediated responses in adult rat dorsal root ganglion cells in vitro. Br. J. Pharmacol. 133:13–22.
Gammon, C. M., Allen, A. C., and Morell, P. 1989. Bradykinin stimulates phosphoinositide hydrolysis and mobilization of arachidonic acid in dorsal root ganglion neurons. J. Neurochem. 53:95–101.
del Rio, E., Bevilacqua, J. A., Marsh, S. J., Halley, P., and Caulfield, M. P. 1999. Muscarinic M1 receptors activate phosphoinositide turnover and Ca2+ mobilization in rat sympathetic neurones, but this signaling pathway does not mediate M-current inhibition. J. Physiol. 520 (Pt 1):101–111.
Washizaki, K., Smith, Q. R., Rapoport, S. I., and Purdon, A. D. 1994. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. J. Neurochem. 63: 727–736.
Grange, E., Deutsch, J., Smith, Q. R., Chang, M., Rapoport, S. I., and Purdon, A. D. 1995. Specific activity of brain palmitoyl-CoA pool provides rates of incorporation of palmitate in brain phospholipids in awake rats. J. Neurochem. 65:2290–2298.
Pestonjamasp, V. K. and Burstein, S. H. 1998. Anandamide synthesis is induced by arachidonate mobilizing agonists in cells of the immune system. Biochim. Biophys. Acta 1394:249–260.
DeMar, J. C. J., Wensel, T. G., and Anderson, R. E. 1996. Biosynthesis of the unsaturated 14–carbon fatty acids found on the N termini of photoreceptor-specific proteins. J. Biol. Chem. 271:5007–5016.
Lehninger, A. L., Nelson, D. L., and Cox, M. M. 1993. Principles of biochemistry, 2nd ed. Worth Press, New York, p. 1090.
Cunnane, S. C., Belza, K., Anderson, M. J., and Ryan, M. A. 1998. Substantial carbon recycling from linoleate into products of de novo lipogenesis occurs in rat liver even under conditions of extreme dietary deficiency. J. Lipid Res. 39:2271–2276.
Rapoport, S. I. 2001. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 16:243–261.
Bazan, N. G., Aveldano de Caldironi, M. I., and Rodriguez de Turco, E. B. 1981. Rapid release of free arachidonic acid in the central nervous system due to stimulation. Prog. Lipid Res. 20:523–529.
Rabin, O., Chang, M. C. J., Grange, E., Bell, J. M., Rapoport, S. I., Deutsch, J., and Purdon, A. D. 1998. Selective acceleration of arachidonic acid reincorporation into brain membrane phospholipid following transient ischemia in awake gerbil. J. Neurochem. 70:324–334.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Purdon, A.D., Rosenberger, T.A., Shetty, H.U. et al. Energy Consumption by Phospholipid Metabolism in Mammalian Brain. Neurochem Res 27, 1641–1647 (2002). https://doi.org/10.1023/A:1021635027211
Issue Date:
DOI: https://doi.org/10.1023/A:1021635027211