Abstract
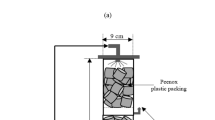
The gaseous streams containing hydrogen sulphide (H2S) mixed with nitrogen gas (N2) (H2S, 0.00145 mol L-1), H2S mixed with liquefied petroleum gas (LPG) and refinery fuel gas were evaluated, in batch operation, in a bubbled column reactor for desulphurization using ferric sulphate as an oxidant. Further, the ferrous sulphate produced in the process of oxidation is biologically oxidized to ferric sulphate using biomass enriched with Thiobacillus ferrooxidans-JSPR1 in flask culture experiments. In all the cases, the gases were bubbled into the biologically generated ferric sulphate (from ferrous sulphate solution) for the oxidation of H2S. The results indicate that0.00426 mol L-1 of ferric ions are required for reacting with0.00145 mol L-1 of H2S in a gaseous stream containing mixture of N2 and H2S. A concentration of 0.00447 mol L-1 of ferric ions is needed for oxidation of 0.00145 mol L-1 of H2S mixed with LPG. Similarly, the refinery fuel gas containing 0.0031 mol L-1 of H2S requires 0.00428 mol L-1 of ferric ion for effective desulphurization. The ratio of moles of H2S reacted to moles of Fe2+ produced at optimal condition was 0.533, 0.516, 0.510, respectively, for nitrogen mixed H2S, LPG mixed H2S and refinery fuel gas containing H2S. The removal of H2S from these gaseous streams was more than 98%sulphate produced in the process could be biologically oxidized to ferric sulphate with an efficiency of 98%, using shake flask culture experiments. Based on flask culture experiments for biooxidation of commercial ferrous sulphate to generate ferric sulphate, the biokinetic constants viz. yield coefficient y, maximum specific growth rate constant μmax and half saturation rate constant K s were evaluated. The yield coefficient was found to be 0.112 while μmax and K s were observed to be 0.1686 hr-1 and 187.9 mg L-1, respectively. The evaluation of biokinetic constants for bio-oxidation of ferrous sulphate generated during the scrubbing of refinery fuel gas containing H2S indicated the value of μmax and K s as 0.1426 hr-1 and 205 mg L-1, respectively. The value of yield coefficient in this real system was found to be 0.102.
Similar content being viewed by others
References
APHA, AWWA: 1995, Standard Methods for the Examination of Water and Wastewater, 19th ed., Washington D.C.
Asai, Satrou, Kouishi, Yasuhiro and Yabu, Tadahiro: 1990, ‘Kinetics of absorption of hydrogen sulphide in aqueous ferric sulphate solution’, AIChE J. 36(9), 1331—1338.
Astaria, G., Savage, D. W. and Bisio, A.: 1983, Gas Treating with Chemical Solvents, John Wiley & Sons, New York.
Bee Le, R., Lefievre, V. and Pavie, P.: 1995, ‘Solutions to VOC and odour emission by adsorption process’, Odours VOCs J. 1, 96—97.
Dalymple, D. A., Trofe, T. W. and Evans, J. M.: 1988, ‘Sulfur Removal through Liquid Redox Chemistry’, paper presented at the Symposium, Opportunities in Synfuel Industry, Biomark, ND.
Department of Biotechnology Ministry of Science and Technology: 1993, Microbial Desulphurization of Fossil Fuels (Coal Oil and Gas), Goverment of India, New Delhi and NEERI, Nagpur.
Gerold, L.: 1965, ‘Chemical Determination of Tyrosine and Protein by the Folin — Ciocalteu Reaction’ in Experimental Biochemistry — A Laboratory Manual, John Wiley & Sons, Inc., London, pp. 149.
Gianna, R., Galileo, M. and Robertiello: 2002, ‘Abbatimento chimico biologico di H2S in correntigassose’, Chimica e Industria 84, 39—45.
Guido, B. and Chiara, P.: 2003, ‘Technologies for the abatement of sulphide compounds from gaseous streams: A comparative overview’, J. Loss Prev. Proc. Industr. 16, 363—371.
Halfmier, H., Schafer, Treffenfeldt, W. and Reus, M.: 1993, ‘Potential of Thiobacillus ferrooxidans for waste gas purification, Part 2, Increase in continuous ferrous iron oxidation kinetics using immobilized cells’, Appl. Microbiol. Biotechnol. 40, 582—587.
Hardison, L. C.: 1985, ‘Go from H2S to S in one unit’, Hydrocarbon Processing 75, 38—40.
Harrison, R. M. and Perry, R.: 1986, Handbook of Air Pollution Analysis, 2nd ed., Chapman and Hall, New York.
Heguy, D. L. and Nagl, G. J.: 2003, ‘Consider optimised iron redox processes to remove sulphur’, Hydrocarbon Processing 82, 53—57.
Hikita, H. and Asai, S.: 1964, ‘Gas absorption with (m,n)-th order irreversible chemical reaction’, Kagaku Kogaku 27, 823; Int. Chem. Eng. 4, 332.
Lizama, H. and Suzuki, I.: 1989, ‘Synergistic competitive inhibition of ferrous ion oxidation by Thiobacillus ferrooxidans by increasing concentration of ferric ion and cells’, Appl. Environ.Microbiol. 55, 2588—2592.
Malhotra, S., Tankhiwale, A. S., Rajvaidya, A. S. and Pandey, R. A.: 2002, ‘Optimal conditions for bio-oxidation of ferrous ions to ferric ions using Thiobacillus ferrooxidans’, Bioresour. Technol. 85, 225—234.
Metcalf and Eddy: 1990, Wastewater Engineering Treatment Disposal Reuse, Tata McGraw-Hill Inc., 2nd ed., 9th reprint, Tata McGraw-Hill Publications, India.
Ministry of Nonconventional Energy Sources: 2002, Microbial Desulphurization of Biogas on Pilot Scale, Goverment of India, New Delhi and NEERI, Nagpur.
Neumann, D. W.: 1984, ‘Oxidative absorption of H2S and O2 by iron chelate solutions’, AICHE J. 1, 62—69.
Pandey, R. A. and Malhotra, S.: 1999, ‘Desulphurization of gaseous fuels with recovery of elemental sulphur: An overview’, Crit. Rev. Environ. Sci. Technol. 29, 229—268.
Satoh, H., Yoshizawa, J. and Kametani, S.: 1988, ‘Bacteria help desulphurize gas’, Hydrocarbon Processing 76, 80—82.
Semt, E., Lens, P. and Langenhove, V. H.: 1998, ‘Treatment of waste gases contaminated with odorous sulphur compounds’, Crit. Rev. Environ. Sci. Technol. 28, 89—117.
Sublette, K. L.: 1987, ‘Aerobic oxidation of hydrogen sulphide by Thiobacillus denitrificans’, Biotech. Bioengg. 29, 690—696.
Turk, A., Sakalis, E., Lessuck, J., Karamitsos, H. and Rago, O.: 1989, ‘Ammonia injection enhances capacity of activated carbon for hydrogen sulfide and methyl mercaptan’, Environ. Sci. Technol. 23, 1242—1245.
U.S. EPA (U.S. Environmental Protection Agency): 1978, U.S. EPA 6001 6-78-074, Washington, D.C.
Wilson, B. M. and Newell, R. D.: 1984, ‘H2S removal by Stretford process — Further development by British Gas Corporation’, presented at National AICHE Meeting Atlanta, GA’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, R.A., Malhotra, S., Rajvaidya, A.S. et al. Chemo-biochemical Desulphurization of Various Gaseous Streams on Bench Scale. Water, Air, & Soil Pollution 154, 295–311 (2004). https://doi.org/10.1023/B:WATE.0000022974.58662.70
Issue Date:
DOI: https://doi.org/10.1023/B:WATE.0000022974.58662.70