Abstract
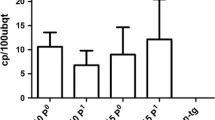
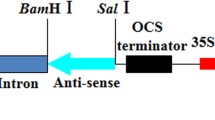
Peanut (Arachis hypogaea L.) lines exhibiting high levels of resistance to peanut stripe virus (PStV) were obtained following microprojectile bombardment of embryogenic callus derived from mature seeds. Fertile plants of the commercial cultivars Gajah and NC7 were regenerated following co-bombardment with the hygromycin resistance gene and one of two forms of the PStV coat protein (CP) gene, an untranslatable, full length sequence (CP2) or a translatable gene encoding a CP with an N-terminal truncation (CP4). High level resistance to PStV was observed for both transgenes when plants were challenged with the homologous virus isolate. The mechanism of resistance appears to be RNA-mediated, since plants carrying either the untranslatable CP2 or CP4 had no detectable protein expression, but were resistant or immune (no virus replication). Furthermore, highly resistant, but not susceptible CP2 T0 plants contained transgene-specific small RNAs. These plants now provide important germplasm for peanut breeding, particularly in countries where PStV is endemic and poses a major constraint to peanut production.
Similar content being viewed by others
References
Cassidy BG and Nelson RS (1995) Differences in protection phenotypes in tobacco plants expressing coat protein genes from peanut stripe potyvirus with and without an engineered ATG. Mol Plant Microbe Interact 8: 357–365.
Chu PWG, Anderson BJ, Khan MRI, Shukla D and Higgins TJV (1999) Production of Bean yellow mosaic virus resistant subterranean clover (Trifolium subterraneum) plants by transformation with the viral coat protein gene. Ann Appl Biol 135: 469–480.
Clark MF and Adams AN (1977) Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol 34: 475–483.
Culver JN, Sherwood JC and Melouk HA (1987) Resistance to peanut stripe virus in Arachis germplasm. Plant Dis 71: 1080–1082.
Demski JW, Reddy DVR, Sowell Jr G and Bays D (1984) Peanut stripe virus – a new seed-borne potyvirus from China infecting groundnut (Arachis hypogaea). Ann Appl Biol 105: 495–501.
Fang G and Grumet R (1993) Genetic engineering of potyvirus resistance using constructs derived from the zucchini yellow mosaic virus coat protein gene. Mol Plant Microbe Interact 6: 358–367.
Finer JJ, Vain P, Jones MWand McMullen MD (1992) Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep 11: 323–328.
Fitch MMM, Manshardt RM, Gonsalves D, Slightom JL and Sanford JC (1992) Virus resistant papaya plants derived from tissues bombarded with the coat protein gene of papaya ringspot virus. Biotechnology 10: 1466–1472.
Graham G, Mayers P and Henry RJ (1994) A simplified method for the preparation of fungal genomic DNA for PCR and RAPD amplification. BioTechniques 16: 48–50.
Han Y and Grierson D (2002) Relationship between small antisense RNAs and aberrant RNAs associated with sense transgene mediated gene silencing in tomato. Plant J 29: 509–519.
Higgins CM and Dietzgen RG (2000) Genetic transformation, regeneration and analysis of transgenic peanut. ACIAR Technical Report No. 48. Australian Centre for International Agricultural Research, Canberra.
Higgins CM, Dietzgen RG, Mat Akin H, Sudarsono, Chen K and Xu Z (1999) Biological and molecular variability of peanut stripe potyvirus. Curr Topics Virol 1: 1–26.
Higgins CM, Hall RM, Campbell PR and Dietzgen RG (2000) PCR rescue and analysis of transgene sequences directly from crude extracts of transgenic embryos and plants. Plant Mol Biol Rep 18: 285.
ICRISAT (1988) Coordination of Research on Peanut Stripe Virus: Research on Peanut Stripe Virus Disease of Groundnut, 9–12 June 1987. Malang, Indonesia. ICRISAT, Pantcheru, India.
Ingelbrecht IL, Irvine JE and Mirkov TE (1999) Posttranscriptional gene silencing in transgenic sugarcane. Dissection of homologydependent virus resistance in a monocot that has a complex polyploid genome. Plant Physiol 119: 1187–1198.
James C (1998) Global review of commercialized transgenic crops. In: ISAAA Briefs No. 8. ISAAA, Ithaca, NY.
Jan F-J, Pang S-Z, Fagoaga C and Gonsalves D (1999) Turnip mosaic potyvirus resistance in Nicotiana benthamiana derived by post-transcriptional gene silencing. Transgenic Res 8: 203–213.
Jones AL, Johansen IE, Bean SJ, Bach I and Maule AJ (1998) Specificity of resistance to pea seed-borne mosaic potyvirus in transgenic peas expressing the viral replicase (Nlb) gene. J Gen Virol 79: 3129–3137.
Kalantidis K, Psaradakis S, Tabler M and Tsagris M (2002) The occurrence of CMV specific short RNAs in transgenic tobacco expressing virus derived dsRNA is indicative of resistance to the virus. Mol Plant Microbe Interact 15: 826–833.
Kochert G (1996) Molecular markers and genome mapping. In: Darussamin A (ed), Current Status of Agricultural Biotechnology in Indonesia. (pp. 89–108). Agency for Agricultural Research and Development, Jakarta.
Li Z, Jarret RL and Demski JW (1997) Engineered resistance to tomato spotted wilt virus in transgenic peanut expressing the viral nucleocapsid gene. Transgenic Res 6: 297–305.
Lindbo JA and Dougherty WG (1992) Pathogen-derived resistance to a potyvirus: immune and resistant phenotypes in transgenic tobacco expressing altered forms of a potyvirus coat protein nucleotide sequence. Mol Plant Microbe Interact 5: 144–153.
Livingstone MJ and Birch RG (1999) Efficient transformation and regeneration of diverse cultivars of peanut (Arachis hypogaea) by particle bombardment into embryogenic callus produced from mature seeds. Mol Breed 5: 43–51.
Lomonossoff GP (1995) Pathogen-derived resistance to plant viruses. Annu Rev Phytopathol 33: 323–343.
Magbanua ZV, Wilde HD, Roberts JK, Chowdhury K, Abad J, Moyer JW et al. (2000) Field resistance to tomato spotted wilt virus in transgenic peanut (Arachis hypogaea L.) expressing an antisense nucleocapsid gene sequence. Mol Breed 6: 227–236.
Mehan VK, Liao BS, Tan YJ, Robinson-Smith A, McDonald D and Hayward AC (1994) Bacterial wilt of groundnut. Info Bull No. 35, ICRISAT, India.
Restrepo MA, Freed, DD and Carrington JC (1990) Nuclear transport of plant potyviral proteins. Plant Cell 2: 987–998.
Revers F, Le Gall O, Candresse T and Maule AJ (1999) New advances in understanding the molecular biology of plant/potyvirus interactions. Mol Plant Microbe Interact 12: 367–376.
Rohini VK and Rao KS (2001) Transformation of peanut (Arachis hypogaea L.) with tobacco chitinase gene: variable response of transformants to leaf spot disease. Plant Sci 160: 889–898.
Sambrook J, Fritsch EF and Maniatis T (1989) Molecular Cloning, a Laboratory Manual. 2nd edn, Cold Spring Harbour Laboratory Press, New York.
Scorza R, Callahan A, Levy L, Damsteegt V, Webb K and Ravelondro M (2001) Post-transcriptional gene silencing in plum pox virus resistant transgenic European plum containing the plum pox potyvirus coat protein gene. Transgenic Res 10: 201–209.
Singit C, Adang MC, Lynch RE, Anderson WA, Wang A, Cardineau G et al. (1997) Expression of a Bacillus thuringiensis cryIA gene in transgenic peanut plants and its efficacy against lesser cornstalk borer. Transgenic Res 6: 169–176.
Smith HA, Powers H, Swaney S, Brown C and Dougherty WG (1995) Transgenic potato virus Y resistance in potato: evidence for an RNA-mediated cellular response. Phytopathology 85: 864–870.
Sonoda S, Mori M and Nishigushi M (1999) Homology-dependent virus resistance in transgenic plants with the coat protein gene of sweet potato feathery mottle potyvirus: target specificity and transgene methylation. Phytopathology 89: 385–391.
Teycheney P-Y and Dietzgen RG (1994) Cloning and sequence analysis of the CP gene of an Australian strain of peanut mottle and an Indonesian 'blotch' strain of peanut stripe potyviruses. Virus Res 31: 235–244.
Urcuqui-Inchima S, Haenni A-L and Bernardi F (2001) Potyvirus proteins: a wealth of functions. Virus Res 74: 157–175.
Waterhouse PM, Wang M-B and Lough T (2001) Gene silencing as an adaptive defence against viruses. Nature 411: 834–842.
Wongkaew S and Dollet M (1990) Comparison of peanut stripe virus strains using symptomatology on particular hosts and serology. Oleagineaux 45: 267–278.
Xu Z, Chen K, Zhang Z and Chen J (1991) Seed transmission of peanut stripe virus in peanut. Plant Dis 75: 723–726.
Yang H, Singsit C, Wang A, Gonsalves D and Ozias-Akins P (1998) Transgenic peanut plants containing a nucleocapsid protein gene of tomato spotted wilt virus show divergent levels of gene expression. Plant Cell Reps 17: 693–699.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higgins, C.M., Hall, R.M., Mitter, N. et al. Peanut Stripe Potyvirus Resistance in Peanut (Arachis Hypogaea L.) Plants Carrying Viral Coat Protein Gene Sequences. Transgenic Res 13, 59–67 (2004). https://doi.org/10.1023/B:TRAG.0000017166.29458.74
Issue Date:
DOI: https://doi.org/10.1023/B:TRAG.0000017166.29458.74