Abstract
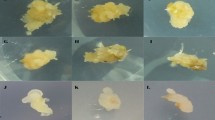
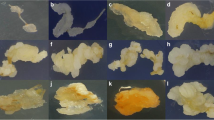
Optimal callus induction and plant regeneration were obtained in bread and durum wheat by manipulating the NaCl concentration in the induction medium. Immature embryos from a high regeneration line of spring wheat (Triticum aestivum L.), 'MPB-Bobwhite 26', and an elite durum wheat (Triticum turgidum var. durum L.), 'Mexicali', were cultured in E3 induction medium consisting of Murashige and Skoog (MS) medium, 2.5 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D), 2% sucrose and 0.9% Bacto agar. The treated embryos were transferred to E3 liquid medium supplemented with various levels of 2,4-D and NaCl. Incubation on medium containing 2.5 mg l−1 2,4-D for 45 days produced callus and plant regeneration in 'MPB-Bobwhite 26', but lower callus yield and plant regeneration in 'Mexicali', indicating that 2,4-D alone was not sufficient for callus induction and plant regeneration in this durum variety. Callus yield and regeneration frequencies were higher in 'Mexicali' embryos that were incubated in media containing 2 mg l−1 2,4-D and 2 mg l−1 NaCl. The presence of NaCl in the medium beyond the initiation phase was detrimental to plant regeneration. The use of NaCl in the callus formation could form the basis for improved transformation of durum wheat varieties.
Similar content being viewed by others
References
Arzani A & Mirodjagh SS (1999) Response of durum wheat cultivars to immature embryo culture, callus induction and in vitro salt stress. Plant Cell Tiss. Org. Cult. 58: 67–72
Barro F, Cannell ME, Lazzeri PA & Barcelo P (1998) The influence of auxins on transformation of wheat and tritordeum and analysis of transgene integration patterns in transformants. Theor. Appl. Gen. 97(5/6): 684–695
Benkirane H, Sabounji K, Chlyah A & Chlyah H (2000) Somatic embryogenesis and plant regeneration from fragments of immature inflorescences and coleoptiles of durum wheat. Plant Cell Tiss. Org. Cult. 61: 107–113
Caswell K, Leung N & Chibbar RN (2000) An efficient method for in vitro regeneration from immature inflorescence explants of Canadian wheat cultivars. Plant Cell Tiss. Org. Cult. 60: 69–73
Delporte F, Mostade O & Jacquemin JM (2001) Plant regeneration through callus initiation from thin mature embryo fragments of wheat. Plant Cell Tiss. Org. Cult. 67: 73–80
Folling L & Olesen A (2001) Transformation of wheat (Triticum aestivum L.) microspore-derived callus and microspores by particle bombardment. Plant Cell Rep. 20: 629–636
Harvey A, Moisan L, Lindup S & Lonsdale D (2001) Wheat regenerated from scutellum callus as a source of material for transformation. Plant Cell Tiss. Org. Cult. 57: 153–156
Littell RA, Milliken GA, Stroup WW & Wolfinger RD (1996) SAS System for Mixed Models. SAS Institute Inc., Cary, NC
Menke-Milczarek I & Zimny J (2001) NH4 + and NO3 - requirement for wheat somatic embryogenesis. Acta Physiol. Plant. 23: 37–42
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Pastori GM, Wilkinson MD, Steele SH, Sparks CA, Jones HD & Parry MA (2002) Age-dependent transformation frequency in elite wheat varieties. J. Exp. Bot. 52: 857–863
Pellegrineschi A, Brito RM, Velazquez L, Noguera LM, Pfeiffer W, McLean S & Hoisington D (2002a) The effect of pretreatment with mild heat and drought stresses on the explant and biolistic transformation frequency of three durum wheat cultivars. Plant Cell Rep. 20: 955–960
Pellegrineschi A, Noguera LM, Skovmand B, Brito RM, Velazquez L, Salgado MM, Hernandez R, Warburton M & Hoisington D (2002b) Identification of highly transformable wheat genotypes for mass production of fertile transgenic plants. Genome 45: 421–430
Weir B, Gu X, Bo WM, Upadhyaya N, Elliott AR & Brettell RIS (2001) Agrobacterium tumefaciens-mediated transformation of wheat using suspension cells as a model system and green fluorescent protein as a visual marker. Aust. J. Plant Physiol. 28: 807–818
Wright M, Dawson J, Dunder E, Suttie J, Reed J, Kramer C, Chang Y, Novitzky R, Wang H & Artim-Moore L (2001) Efficient biolistic transformation of maize (Zea mays L.) and wheat (Triticum aestivum L.) using the phosphomannose isomerase gene, pmi, as the selectable marker. Plant Cell Rep. 20: 429–436
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pellegrineschi, A., Brito, R.M., McLean, S. et al. Effect of 2,4-Dichlorophenoxyacetic Acid and NaCl on the Establishment of Callus and Plant Regeneration in Durum and Bread Wheat. Plant Cell, Tissue and Organ Culture 77, 245–250 (2004). https://doi.org/10.1023/B:TICU.0000018389.99656.d8
Issue Date:
DOI: https://doi.org/10.1023/B:TICU.0000018389.99656.d8