Abstract
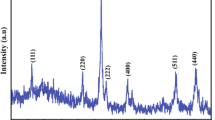
We have used X-ray phase analysis, transmission electron microscopy, and temperature-programmed reduction to study cobalt–zirconium catalysts. The most active samples are characterized by higher dispersity of both the support (L = 12-13 nm) and the active component (L ≤ 3 nm); modification of the zirconium dioxide with yttrium oxide increases the thermal stability of the catalysts. We have shown that the activity of the catalysts is determined by both the strength of oxygen–catalyst binding and the acidic properties of the catalyst surface.
Similar content being viewed by others
REFERENCES
M. Campanati, G. Fornasari, and A. Vaccari, Catal. Today, 77, 299-314 (2003).
S. N. Orlik, M. R. Kantserova, K. S. Gavrilenko, and G. R. Kosmambetova, Nanosist., Nanomater., Nanotekhnol., 1, No. 2, 529-550 (2003).
Y. Li and J. N. Armor, Appl. Catal. B., 3, No. 4, 275-282 (1994).
A. N. Kharlanov, E. V. Lunina, and V. V. Lunin, Zh. Fiz. Khim., 73, No. 5, 898-904 (1999).
V. G. Milt, M. A. Ulla, and E. A. Lombardo, Catal. Lett., 65, Nos. 1-3, 67-73 (2000).
V. R. Choudhary, S. Banerjee, and B. S. Uphade, Appl. Catal. A., 197, No. 2, L183-186 (2000).
Yu. M. Rodionov, E. M. Slyusarenko, and V. V. Lunin, Usp. Khim., 65, No. 9, 865-880 (1996).
A. S. Ivanova, Zh. Prikl. Khim., 69, No. 11, 1790-1800 (1996).
M. Labaki, J.-F. Lamonier, S. Siffert, et al., Abstracts, Sixth Russian Conference on Mechanisms of Catalytic Reactions, Moscow, 2002 [in Russian], Inst. Katal. SO RAN, Novosibirsk (2002), pp. 51-52.
V. A. Dzis'ko, A. P. Karnaukhov, and D. V. Tarasova, Physicochemical Principles for Synthesis of Oxide Catalysts [in Russian], Nauka, Novosibirsk (1978).
N. F. Uvarov and V. V. Boldyrev, Usp. Khim., 70, No. 4, 307-329 (2001).
R. Burch, D. J. Crittle, and M. J. Hayes, Catal. Today, 47, Nos. 1-4, 229-234 (1999).
V. R. Choudhary and V. H. Rane, J. Catal., 130, No. 2, 411-417 (1991).
Hsiao-Chung Wu, Liang-Chi Liu, and Sze-Ming Yang, Appl. Catal. A., 211, No. 2, 159-165 (2001).
J. Yan, M. C. Kung, W. M. H. Sachtler, et al., J. Catal., 175, No. 2, 294-303 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kantserova, M.R., Orlik, S.N. & Kazimirov, V.P. Effect of the Structure and Size Factor on the Catalytic Properties of Cobalt–Zirconium Oxide Nanoparticles in Deep Oxidation of Methane. Theoretical and Experimental Chemistry 40, 246–253 (2004). https://doi.org/10.1023/B:THEC.0000041810.48201.4f
Issue Date:
DOI: https://doi.org/10.1023/B:THEC.0000041810.48201.4f