Abstract
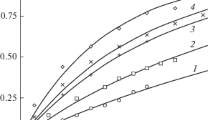
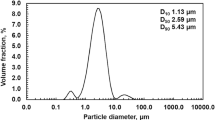
A detailed analysis of the kinetics of iron(II, III) oxide dissolution in hydrochloric acid is performed using a generalized Delmon model, which takes into account changes in the fractal dimension of the surface of dissolving particles.
Similar content being viewed by others
REFERENCES
Seo, M. and Sato, N., The Dissolution of Hydrous Metal Oxides in Acid Solutions, Boshoku Gijutsu (Corr. Eng.), 1975, vol. 24, p. 399; 1976, vol. 25, p. 161.
Gorichev, I.G. and Kipriyanov, N.A., Kinetics of Dissolution of Oxide Phases in Acids, Zh. Fiz. Khim., 1981, vol. 55, no. 11, p. 2734.
Gorichev, I.G. and Kipriyanov, N.A., Kinetics of Dissolution of Metal Oxides in Acidic Media, Usp. Khim., 1984, vol. 53, no. 11, p. 1790.
Frenier, W.W. and Growcock, F.B., Mechanism of Iron Oxide Dissolution: Review of Recent Literature, Corrosion (NACE), 1984, vol. 40, no. 12, p. 663.
Blesa, M.A. and Maroto, A.J.G., Dissolution of Metal Oxides, J. Chim. Phys., 1986, vol. 83, nos. 11/12, p. 757.
Batrakov, V.V., Gorichev, I.G., and Kipriyanov, N.A., Effect of the Electrical Double Layer on the Kinetics of Dissolution of Metal Oxides, Elektrokhimiya, 1994, vol.?30, no. 4, p. 444.
Bruyere, V.I.E. and Blesa, M.A., Acidic and Reductive Dissolution of Magnetite in Aqueous Sulfuric Acid, J.?Electroanal. Chem., 1985, vol. 182, p. 141.
Aquatic Chemical Kinetics: Reaction Rates of Processes in Natural Waters, Stumm, W., Ed., New York: Wiley, 1990, p. 650.
Dyatlova, N.M. and Gorichev, I.G., Effect of Chelators on the Kinetics of Dissolution of Metal Oxides, Koord. Khim., 1986, vol. 12, no. 1, p. 3.
Delmon, B., Introduction a la cinetique heterogene, Paris: Technip, 1969. Translated under the title Kinetika geterogennykh reaktsii, Moscow: Mir, 1972.
Rozovskii, A.Ya., Geterogennye khimicheskie reaktsii(Heterogeneous Chemical Reactions)}, Moscow: Nauka, 1980.
Brown, M.E., Dollimore, D., and Galwey, A.K., Reactions in the Solid State, vol. 22 of Comprehensive Chemical Kinetics, Bamford, C.H. and Tipper, C.F.H., Eds., Amsterdam: Elsevier, 1980. Translated under the title Reaktsii tverdykh tel, Moscow: Mir, 1983.
Young, D.A., Decomposition of Solids, Oxford: Pergamon, 1966. Translated under the title Kinetika razlozheniya tverdykh veshchestv, Moscow: Mir, 1969.
Barret, P., Cinetique heterogene, Paris: Gauthier-Villars, 1973. Translated under the title Kinetika geterogennykh protsessovPeople's/E Moscow: Mir, 1976.
Chastukhin, A.E., Izotov, A.D., Gorichev, I.G., and Kutepov, A.M., Analysis of Fe2O3 and Fe3O4 Dissolution Kinetics in Terms of the Chain Mechanism Model, Teor. Osn. Khim. Tekhnol., 2003, vol. 37, no. 4, p. 427.
Marczenko, Z., Kolorymetryczne oznaczanie pierwiastkyw(Colorimetric Determination of Elements)}, Warsaw: Wydawn. Nauk.-Tech., 1968. Translated under the title Fotometricheskoe opredelenie elementov, Moscow:Mir, 1971.
Gorichev, I.G., Izotov, A.D., Ilyukhin, O.V., and Kutepov, A.M., An Analysis of Kinetic Data on Solution of Metal Oxides in Terms of Fractal Geometry, Zh. Fiz. Khim., 1999, vol. 73, no. 10, p. 1802.
Kometiani, Z.P. and Vekua, M.G., Kinetika membrannykh transportnykh fermentov(Kinetics of Membrane Transport Enzymes)}, Moscow: Vysshaya Shkola, 1988.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chastukhin, A.E., Izotov, A.D., Gorichev, I.G. et al. Analysis of the Kinetics of Iron(II, III) Oxide Dissolution in Hydrochloric Acid Using a Generalized Delmon Model. Theoretical Foundations of Chemical Engineering 38, 81–85 (2004). https://doi.org/10.1023/B:TFCE.0000014393.78680.a7
Issue Date:
DOI: https://doi.org/10.1023/B:TFCE.0000014393.78680.a7