Abstract
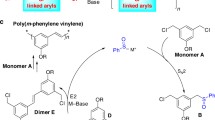
Lithiation of various substrates, such as chlorinated acetals, α-chloro ether, dichloro derivatives benzo-fused heterocycles, and allyl and benzyl derivatives, with excess lithium powder in the presence of a catalytic amount of soluble linear or insoluble cross-linked arene (naphthalene or biphenyl)-based polymers yields the expected organolithium intermediates. The latter react with electrophiles either in two steps or under Barbier-type reaction conditions to afford the corresponding adducts. The catalyst is easily recuperated by filtration at the end of the process, and the procedure can be regarded as a reasonable alternative to the use of free arenes as electron carrier in lithiation reactions.
Similar content being viewed by others
REFERENCES
Boudier, A., Bromm, L.O., Lotz, L., and Knochel, P., Angew. Chem. Int. Ed., 2000, vol. 39, p. 4414.
Najera, C. and Yus, M., Trends Org. Chem., 1991, vol. 2, p. 155; Najera, C. and Yus, M., Recent Res. Dev. Org. Chem., 1997, vol. 7, p. 67; Najera, C. and Yus, M., Curr. Org. Chem., 2003, vol. 7, p. 867.
Wakefield, B.J., Organolithium Methods, London: Academic, 1988; Lithium Chemistry. A Theoretical and Experimental Overview, Sapse, A.-M. and Schleyer, P.v.R., Eds., Chi-chester: Wiley, 1995; Bartsab, R., Drost, C., and Klingebiel, U., Synthetic Methods of Organometallic and Inorganic Chemistry, Herrmann, W.A., Ed., Stuttgart: Georg Thieme, 1996, vol. 2; Clayden, J., Organolithiums: Selectivity for Synthesis, Oxford: Pergamon, 2002.
Yus M. and Ramón, D.J., J. Chem. Soc., Chem. Commun., 1991, p. 398.
Yus, M., Chem. Soc. Rev., 1996, vol. 25, p. 155; Ramón, D.J. and Yus, M., Eur. J. Org. Chem., 2000, p. 225; Yus, M., Synlett, 2001, p. 1197; Yus, M. and Ramón, D.J., J. Latv. Chem., 2002, p. 79; Ramón, D.J. and Yus, M., Rev. Cubana Quim., 2002, vol. 14, p. 75; Yus, M., The Chemistry of Organolithium Compounds, Rappoport, Z. and Mareck, I., Eds., Chichester: Wiley, 2003, in press.
Yus, M., Herrera, R.P., and Guijarro, A., Tetrahedron Lett., 2001, vol. 42, p. 3455; Yus, M., Herrera, R.P., and Guijarro, A., Chem. Eur.J., 2002, vol. 8, p. 2574; Herrera, R.P., Guijarro, A., and Yus, M., Tetrahedron Lett., 2003, vol. 44, p. 1309; Herrera, R.P., Guijarro, A., and Yus, M., Tetrahedron Lett., 2003, vol. 44, p. 1313; Yus, M., Herrera, R.P., and Guijarro, A., Tetrahedron Lett., 2003, vol. 44, p. 5025.
Holy, N.L., Chem. Rev., 1974, vol. 74, p. 243.
Guijarro, D. and Yus, M., Recent Res. Dev. Org. Chem., 1998, vol. 2, p. 713.
Yus, M. and Foubelo, F., Rev. Heteroatom Chem., 1997, vol. 77, p. 73.
Foubelo, F. and Yus, M., Trends Org. Chem., 1998, vol. 7, p. 1.
Cintas, P., Activated Metals in Organic Synthesis, Boca Raton: CRC, 1993; Active Metals, Furstner, A., Ed., Weinheim: VCH, 1996; Furstner, A., Angew. Chem. Int. Ed. Engl., 1993, vol. 32, p. 164; Guijarro, A., Gómez, C. and Yus, M., Trends Org. Chem., 2000, vol. 8, p. 65.
Alonso, F., Vitale, C., Radivoy, G., and Yus, M., Synthesis, 2003, p. 443.
Alonso, F. and Yus, M., Recent Res. Devel. Org. Chem., 1997, vol. 1, p. 397.
Gómez, C., Ruiz, S., and Yus, M., Tetrahedron Lett., 1998, vol. 39, p. 397; Gómez, C., Ruiz, S., and Yus, M., Tetrahedron, 1999, vol. 55, p. 7017; Atnauld, T., Barret, A.G.M., and Hopkins, B.T., Tetrahedron Lett., 2002, vol. 43, p. 1081.
Alonso, F., Candela, P., Gómez, C., and Yus, M., Adv. Synth. Calal., 2003, vol. 345, p. 275.
Yus, M., Gómez, C., and Candela, P., Tetrahedron, 2002, vol. 58, p. 6207.
Yus, M., Gómez, C., and Candela, P., Tetrahedron, 2003, vol. 59, p. 1909.
Gil, J.F., Ramón, D.J., and Yus, M., Tetrahedron, 1993, vol. 49, p. 4923.
Ramón, D.J. and Yus, M., Tetrahedron Lett., 1990, vol. 31, p. 3763; Ramón, D.J. and Yus, M., J. Org. Chem., 1991, vol. 56, p. 3825.
Guijarro, A. and Yus, M., Tetrahedron Lett., 1993, vol. 34, p. 3487; Guijarro, A., Mancheno, B., Ortiz, J., and Yus, M., Tetrahedron, 1996, vol. 52, p. 1643.
Ramón, D.J. and Yus, M., Tetrahedron Lett., 1992, vol. 33, p. 2217; Gómez, C., Ramon, D.J., and Yus, M., Tetrahedron, 1993, vol. 49, p. 4117; Alonso, F., Lorenzo, E., and Yus, M., Tetrahedron Lett., 1997, vol. 38, p. 2187; Lorenzo, E., Alonso, F., and Yus, M., Tetrahedron, 2000, vol. 56, p. 1745.
Gómez, C., Huerta, F.F., and Yus, M., Tetrahedron Lett., 1997, vol. 38, p. 687; Gómez, C., Huerta, F.F., and Yus, M., Tetrahedron, 1998, vol. 54, p. 1853.
Almena, J., Foubelo, F., and Yus, M., Tetrahedron, 1995, vol. 51, p. 3351.
Almena, J., Foubelo, F., and Yus, M., Tetrahedron, 1995, vol. 51, p. 3365.
Alonso, E., Guijarro, D., Martinez, P., Ramón, D.J., and Yus, M., Tetrahedron, 1999, vol. 55, p. 11027.
Alonso, E., Ramón, D.J., and Yus, M., Tetrahedron, 1997, vol. 53, p. 14355.
Yus, M., Martinez, P., and Guijarro, D., Tetrahedron, 2001, vol. 57, p. 10119.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Candela, P., Gómez, C. & Yus, M. Lithiation Reactions Catalyzed by Linear and Cross-Linked Arene-Based Polymers. Generation of Functionalized Organolithium Compounds. Russian Journal of Organic Chemistry 40, 795–801 (2004). https://doi.org/10.1023/B:RUJO.0000044541.84132.e5
Issue Date:
DOI: https://doi.org/10.1023/B:RUJO.0000044541.84132.e5