Abstract
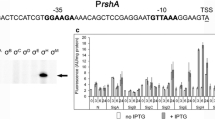
Expression of the microcin C51 operon in Escherichia coli cells is activated during cell entry into the stationary growth phase and depends on the σS subunit of RNA polymerase (RpoS). The null rpoS mutations retained the residual expression level of the transcriptional Pmcc-lac fusion, which indicates that other sigma subunit can participate in the regulation of transcription of the microcin C51 operon. Data presented in this work show that the overproduction of σ70 in rpoS cells diminished the level of Pmcc-lac expression, as in wild-type cells, which seems to be the consequence of competition between sigma factors for a limited number of core RNA polymerase molecules. In the presence of the rpoD800 mutation that renders σ70 temperature-sensitive, expression of Pmcc-lac was not induced in the phase of delayed culture growth at nonpermissive temperature, which indicates that σ70 is indispensable for microcin operon expression. Point substitutions in the –10 Pmcc region, leading to the formation of 5′-TGaTATAAT-3′ site, enhanced promoter activity but did not affect the relationship between Pmcc-lac transcription and growth phase, σS, and the activator protein CRP. The activator protein CRP was shown to bind a DNA fragment containing the TGTGA(AATGAA)TCTAT site in the –59.5 bp position relative to the start site of transcription. Mutation in the ssr1 gene encoding 6S RNA did not disturb Pmcc-lac expression; these results indicate that 6S RNA does not participate in the regulation of microcin C51 operon expression.
Similar content being viewed by others
REFERENCES
Kolter, R., Sigele, D.A., and Tormo, A., The Stationary Phase of the Bacterial Life Cycle, Annu. Rev. Microbiol., 1993, vol. 47, pp. 855–874.
Ishihama, A., Modulation of the Nucleoid, the Transcription Apparatus, and the Translation Machinery in Bacteria for Stationary Phase Survival, Genes Cells, 1999, vol. 4, pp. 135–143.
Hengge-Aronis, R., Signal Transduction and Regulatory Mechanisms Involved in Control of the σS (RpoS) Subunit of RNA Polymerase, Microbiol. Mol. Biol. Rev., 2002, vol. 66, pp. 373–395.
Hengge-Aronis, R., Stationary Phase Gene Regulation: What Makes an Escherichia coli Promoter σS Selective?, Curr. Opin. Microbiol., 2002, vol. 5, pp. 591–595.
Kurepina, N.E., Basyuk, E.I., Metlitskaya, A.Z., et al., Cloning and Mapping of the Genetic Determinants for Microcin C51 Production and Immunity, Mol. Gen. Genet., 1993, vol. 241, pp. 700–706.
Metlitskaya, A.Z., Katrukha, G.S., Shashkov, A.S., et al., Structure of Microcin C51, a New Antibiotic with a Broad Spectrum of Activity, FEBS Lett., 1995, vol. 357, pp. 235–238.
Fomenko, D.E., Metlitskaya, A.Z., Peduzzi, J., et al., Microcin C51 Plasmid Genes: Possible Source of Horizon-tal Gene Transfer, Antimicrob. Agents Chemother., 2003, vol. 47, pp. 2868–2874.
González-Pastor, J.E., San Millán, J.L., Castilla, M.A., and Moreno, F., Structure and Organization of Plasmid Genes Required to Produce the Translation Inhibitor Microcin C7, J. Bacteriol., 1995, vol. 177, pp. 7131–7140.
Loewen, P.C. and Hengge-Aronis, R., The Role of the Sigma Factor σS (KatF) in Bacterial Global Regulation, Annu. Rev. Microbiol., 1994, vol. 48, pp. 53–80.
Fomenko, D., Veselovskii, A., and Khmel, I., Regulation of Microcin C51 Operon Expression: the Role of Global Regulators of Transcription, Res. Microbiol., 2001, vol. 152, pp. 469–479.
Veselovskii, A.M., Fomenko, D.E., Metlitskaya, A.Z., et al., Properties of the Functioning of the Promoter of the Microcin C51 Operon under Different Conditions of Escherichia coli Cell Growth, Russ. J. Genet., 2001, vol. 37, no. 8, pp. 876–883.
Miller, J.H., Experiments in Molecular Genetics, Cold Spring Harbor, New York: Cold Spring Harbor Lab., 1972.
Silhavy, T.J., Berman, M.L., and Enquist, L.W., Experi-ments with Gene Fusions, Cold Spring Harbor, New York: Cold Spring Harbor Lab., 1984.
Simons, R.W., Houman, F., and Kleckner, N., Improved Single and Multicopy lac-Based Cloning Vectors for Protein and Operon Fusions, Gene, 1987, vol. 53, pp. 85–96.
Sambrook, J., Fritsch, E.F., and Maniatis, T., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, New York: Cold Spring Harbor Lab., 1989.
Brikun, I., Suziedelis, K., Stemmann, O., et al., Analysis of CRP–CytR Interactions at the Escherichia coli udp Promoter, J. Bacteriol., 1996, vol. 178, pp. 1614–1622.
Farewell, A., Kvint, K., and Nystrom, T., Negative Regulation by RpoS: A Case of Sigma Factor Competition, Mol. Microbiol., 1998, vol. 29, pp. 1039–1051.
Bedwell, D.M. and Nomura, M., Feedback Regulation of RNA Polymerase Subunit Synthesis after the Condi-tional Overproduction of RNA Polymerase in Escheri-chia coli, Mol. Gen. Genet., 1986, vol. 204, pp. 17–23.
Jenkins, D.E., Auger, E.A., and Matin, A., Role of RpoH, a Heat Shock Regulator Protein, in Escherichia coli Carbon Starvation Protein Synthesis and Survival, J. Bacte-riol., 1991, vol. 173, pp. 1992–1996.
Grossman, A.D., Straus, D.B., Walter, W.A., and Gross, C.A., σ32 Synthesis Can Regulate the Synthesis of Heat Shock Proteins in Escherichia coli, Genes Dev., 1987, vol. 1, pp. 179–184.
Liebke, H., Gross, C., Walter, W., and Burgess, R., A New Mutation rpoD800 Affecting the ΣE. coli RNA Polymerase Is Allelic to Two Other σ Mutants, Mol. Gen. Genet., 1980, vol. 177, pp. 277–282.
Ueno-Nishio, S., Backman, K.C., and Magasanik, B., Regulation at the glnL-Operator-Promoter of the Complex glnALG Operon of Escherichia coli, J. Bacteriol., 1983, vol. 153, pp. 1247–1251.
Jishage, M., Iwata, A., Ueda, S., and Ishihama, A., Regulation of RNA Polymerase σSubunit Synthesis in Escherichia coli: Intracellular Levels of Four Species of Σ Subunit under Various Growth Conditions, J. Bacte-riol., 1996, vol. 178, pp. 5447–5451.
Wassarman, K.M. and Storz, G., 6S RNA Regulates E. coli RNA Polymerase Activity, Cell (Cambridge, Mass.), 2000, vol. 101, pp. 613–623.
Moreno, F., Gσnzalez-Pastor, J.E., Baquero, M.-R., and Bravo, D., The Regulation of Microcin B, C, and J Operons, Biochimie, 2002, vol. 84, pp. 521–529.
Kolb, A., Kotlarz, D., Kusano, S., and Ishihama, A., Selectivity of the Escherichia coli RNA Polymerase EΣ38 for Overlapping Promoters and Ability to Support CRP Activation, Nucleic Acids Res., 1995, vol. 23, pp. 819–826.
Tanaka, K., Kusano, S., Fujita, N., et al., Promoter Determinants for Escherichia coli RNA Polymerase Holoenzyme Containing σ38 (the rpoS Gene Product), Nucleic Acids Res., 1995, vol. 23, pp. 827–834.
Colland, F., Fujita, N., Kotlarz, D., et al., Positioning of σS, the Stationary Phase σFactor, in Escherichia coli RNA Polymerase–Promoter Open Complexes, EMBO J., 1999, vol. 18, pp. 4049–4059.
Lee, S.J. and Gralla, J.D., σ38 (RpoS) RNA Polymerase Promoter Engagement via –10 Region Nucleotides, J. Biol. Chem., 2001, vol. 276, pp. 30 064–30 071.
Kumar, A., Malloch, R.A., Fugita, N., et al., The Minus 35-Recognition Region of Escherichia coli σ70 Is Ines-sential for Initiation of Transcription at an “Extended Minus 10” Promoter, J. Mol. Biol., 1993, vol. 232, pp. 406–418.
Barne, K.A., Bown, J.A., Busby, J.W., and Minchin, S.D., Region 2.5 of the Escherichia coli RNA Polymerase σ70 Subunit Is Responsible for the Recognition of the “Extended –10” Motif at Promoters, EMBO J., 1997, vol. 16, pp. 4034–4040.
Burr, T., Mitchell, J., Kolb, A., et al., DNA Sequence Elements Located Immediately Upstream of the –10 Hexamer in Escherichia coli Promoters: A Systematic Study, Nucleic Acids Res., 2000, vol. 28, pp. 1864–1870.
Wise, A., Brems, R., Ramakrishnan, V., and Villarejo, M., Sequences in the –35 Region of Escherichia coli RpoS-Dependent Genes Promote Transcription by? σS, J. Bacteriol., 1996, vol. 178, pp. 2785–2793.
Bordes, P., Repoila, F., Kolb, A., and Gutierres, C., Involvement of Differential Efficiency of Transcription by EσS and Eσ70 RNA Polymerase Holoenzymes in Growth Phase Regulation of the Escherichia coli osmE Promoter, Mol. Microbiol., 2000, vol. 35, pp. 845–853.
Kolb, A., Busby, S., Buc, H., et al., Transcriptional Regulation by cAMP and Its Receptor Protein, Annu. Rev. Biochem., 1993, vol. 62, pp. 749–795.
Lonetto, M., Gribskov, M., and Gross, C.A., The σ70 Family: Sequence Conservation and Evolutionary Relationships, J. Bacteriol., 1992, vol. 174, pp. 3843–3849.
Schellhorn, H.E., Audia, J.P., Wei, L.I.C., and Chang, L., Identification of Conserved, RpoS-Dependent Station-ary-Phase Genes of Escherichia coli, J. Bacteriol., 1998, vol. 180, pp. 6283–6291.
Altuvia, S., Almiron, M., Huisman, G., et al., The dps Promoter Is Activated by OxyR during Growth and by IHF and σS in Stationary Phase, Mol. Microbiol., 1994, vol. 13, pp. 265–272.
Olsén, A., Arnqvist, A., Hammar, M., et al., The RpoS σ Factor Relieves H-NS-Mediated Transcriptional Repression of csgA, the Subunit Gene of Fibronectin-Binding Curli in Escherichia coli, Mol. Microbiol., 1993, vol. 7, pp. 523–536.
Lange, R., Barth, M., and Hengge-Aronis, R., Complex Transcriptional Control of the σS-Dependent Stationary-Phase-Induced and Osmotically Regulated osmY (csi-5) Gene Suggests Novel Roles for Lrp, Cyclic AMP (cAMP) Receptor Protein–cAMP Complex, and Integra-tion Host Factor in the Stationary-Phase Response of Escherichia coli, J. Bacteriol., 1993, vol. 175, pp. 7910–7917.
Kvint, K., Hosbond, C., Farewell, A., et al., Emergency Derepression: Stringency Allows RNA Polymerase to Override Negative Control by an Active Repressor, Mol. Microbiol., 2000, vol. 35, pp. 435–443.
Colland, F., Barth, M., Hengge-Aronis, R., and Kolb, A., Factor Selectivity of Escherichia coli RNA Polymerase: Role for CRP, IHF and Lrp Transcription Factors, EMBO J., 2000, vol. 19, pp. 3028–3037.
Maeda, H., Fujita, N., and Ishihama, A., Competition among Seven Escherichia coli σSubunits: Relative Binding Affinities to the Core RNA Polymerase, Nucleic Acids Res., 2000, vol. 28, pp. 3497–3503.
Conter, A., Menchon, C., and Gutierrez, C., Role of DNA Supercoiling and RpoS σFactor in the Osmotic and Growth Phase-Dependent Induction of the Gene osmE of Escherichia coli K12, J. Mol. Biol., 1997, vol. 273, pp. 75–83.
Santos, J.M., Freire, P., Vicente, M., and Arraiano, C.M., The Stationary-Phase Morphogene bolA from Escheri-chia coli Is Induced by Stress during Early Stages of Growth, Mol. Microbiol., 1999, vol. 32, pp. 789–798.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Veselovskii, A.M., Bass, I.A., Zolotukhina, M.A. et al. Involvement of Sigma S and Sigma 70 Subunits of RNA Polymerase and the CRP Protein in the Regulation of Microcin C51 Operon Expression. Russian Journal of Genetics 40, 1199–1209 (2004). https://doi.org/10.1023/B:RUGE.0000048661.42211.a0
Issue Date:
DOI: https://doi.org/10.1023/B:RUGE.0000048661.42211.a0