Abstract
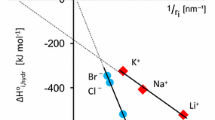
The structural features of concentrated aqueous solutions of MeCl (Me = Li, Na, K, Rb; molar ratio [salt] : [water] = 1 : 15) at 293 K and 0.1 MPa were studied by the method of integral equations. The calculation results show that the disordering effect of the cation on the solvent structure grows in the order NaCl < KCl < RbCl (the number of free water molecules grows, and the content of tetrahedrally ordered molecules decreases). With LiCl, the changes in these parameters are maximal. In the systems containing Li+ and Na+ ions, the association parameter is lower than in pure water, whereas in the solutions with the K+ and Rb+ ions it is higher, in agreement with the concept of positive and negative hydration. It was suggested that, with increasing cationic radius, formation of hydrogen bonds between bulk water molecules becomes more preferential, and interactions between the anion and solvent molecules are weakened. On the contrary, the coordination number of the cation increases with its radius. In the examined series of solutions, the probability of formation of contact ion pairs grows considerably, and that of formation of hydration-separated ion pairs decreases.
Similar content being viewed by others
REFERENCES
Roux, B. and Karplus, M., Ann. Rev. Biophys. Biomol. Struct., 1994, vol. 23, p. 731.
Jordan, P.C., Biophys. J., 1990, vol. 58,no. 5, p. 1133.
Chandler, D. and Andersen, H.C., J. Chem. Phys., 1972, vol. 57,no. 5, p. 1930.
Enderby, J.E. and Neilson, G.W., Adv. Phys., 1980, vol. 29,no. 2, p. 323.
Hummer, G. and Soumpasis, D.M., Mol. Phys., 1992, vol. 77,no. 4, p. 769.
Holovko, M.F. and Kalyuzhnyi, Yu.V., Mol. Phys., 1989, vol. 68,no. 6, p. 1239.
Ohba, M. and Arakawa, K., Bull. Chem. Soc. Jpn., 1985, vol. 58,no. 1, p. 9.
Fedotova, M.V., Cand. Sci. (Chem.) Dissertation, Ivanovo, 1994.
Fedotova, M.V. and Trostin, V.N., Zh. Fiz. Khim., 1996, vol. 70,no. 6, p. 1019.
Fedotova, M.V., Nikologorskaya, E.L., Kuznetsov, V.V., and Trostin, V.N., Zh. Neorg. Khim., 1996, vol. 41,no. 2, p. 326.
Kalyuzhnyi, Yu.V., Fedotova, M.V., Golovko, M.F., and Trostin, V.N., Preprint of the Inst. of the Physics of Condensed Systems, Ukrainian National Acad. Sci., Lviv, 1994, no. 93-27P.
Fedotova, M.V. and Trostin, V.N., Zh. Fiz. Khim., 1996, vol. 70,no. 10, p. 1804.
Smith, D.E. and Dang, L.X., J. Chem. Phys., 1994, vol. 100,no. 5, p. 3757.
Heinzinger, K., Physica B, 1985, vol. 131, p. 196.
Zhu, S.-B. and Robinson, G.W., J. Chem. Phys., 1992, vol. 97,no. 6, p. 4336.
Berendsen, H.J.C., Postma, J.P.M., Van Gunsteren, W.F., and Hermans, J., Jerusalem Symp. on Quantum Chemistry and Biochemistry, Pullman, B., Ed., Dordrecht: Reidel, 1981, p. 331.
Pettitt, B.M. and Rossky, P.J., J. Chem. Phys., 1982, vol. 77,no. 3, p. 1451.
Pettitt, B.M. and Rossky, P.J., J. Chem. Phys., 1986, vol. 84,no. 10, p. 5836.
Dang, L.X., J. Am. Chem. Soc., 1995, vol. 117,no. 26, p. 6954.
Fumi, F.G. and Tosi, M.P., J. Phys. Chem. Solids, 1964, vol. 25,no. 1, p. 31.
Gucker, F.T., Stubley, D., and Hill, D.J., J. Chem. Thermodyn., 1977, vol. 9,no. 10, p. 987.
Litvinenko, I.V., Zh. Strukt. Khim., 1963, vol. 4,no. 6, p. 830.
Celeda, J., Coll. Czech. Chem. Commun., 1983, vol. 48,no. 6, p. 1680.
Zagainov, V.M., Sevryugin, V.A., Alekseeva, S.I., and Emel'yanov, M.I., in Fizika zhidkosti (Fluid Physics), Kazan, 1986, p. 110.
Raukhvarger, L.E., Gorbovitskaya, T.I., Kreitus, I.V., and Timiks, Yu.E., Izv. Akad. Nauk Latv. SSR, Ser. Khim., 1984, no. 9, p. 207.
Muller, K.J. and Hertz, H.G., J. Phys. Chem., 1996, vol. 100,no. 4, p. 1256.
Samoilov, O.Ya., Struktura vodnykh rastvorov elektrolitov i gidratatsiya ionov (Structure of Aqueous Electrolyte Solutions and Ion Hydration), Moscow: Akad. Nauk SSSR, 1957.
Lee, S.H. and Rasaiah, J.C., J. Phys. Chem., 1996, vol. 100,no. 4, p. 1420.
Koneshan, S., Rasaiah, J.C., Lynden-Bell, R.M., and Lee, S.H., J. Phys. Chem., 1998, vol. 102,no. 21, p. 4193.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oparin, R.D., Fedotova, M.V. & Trostin, V.N. Features of Cation Hydration in Concentrated Aqueous Solutions of MeCl (Me = Li, Na, K, Rb): A Study by the Method of Integral Equations. Russian Journal of General Chemistry 74, 13–20 (2004). https://doi.org/10.1023/B:RUGC.0000025166.75929.e4
Issue Date:
DOI: https://doi.org/10.1023/B:RUGC.0000025166.75929.e4