Abstract
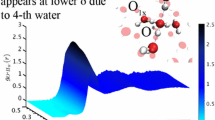
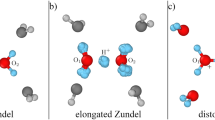
A new mechanism and formalism for proton transfer in donor-acceptor complexes with long hydrogen bonds introduced recently [1], is applied to a proton transfer in liquid water. “Structural diffusion” of hydroxonium ions is regarded as totally adiabatic process, with synchronous hindered translation of two closest water molecules to and from the reaction complex as crucial steps. The water molecules induce a “gated” shift of the proton from the donor to the acceptor in the double-well potential with simultaneous breaking/formation of hydrogen bonds between these molecules and the proton donor and acceptor. The short-range and long-range proton transfer as “structural diffusion” of Zundel complexes is also considered. The theoretical formalism is illustrated with the use of Morse, exponential, and harmonic molecular potentials. This approach is extended to proton transfer in strongly hydrogen-bonded donor-acceptor complexes. In contrast to the above model [1], the short hydrogen bond between the donor and acceptor moieties, however, completely erodes the barrier along the proton transfer mode. This introduces some physical pattern differences from proton transfer reactions in truly double-well potentials with a finite proton transfer barrier at the transition configuration with respect to the environmental nuclear coordinates. The differences apply particularly to the origin of the kinetic isotope effect. We discuss explicitly details of the excess proton conductivity in aqueous solution, but the concepts and formalism apply broadly to acid-base reactions, proton conduction channels, and other strongly hydrogen-bonded O- and N-proton donor-acceptor systems.
Similar content being viewed by others
REFERENCES
Kuznetsov, A.M. and Ulstrup, J., Elektrokhimiya, 2004.
Kornyshev, A.A., Kuznetsov, A.M., Spohr, E., and Ulstrup, J., J. Phys. Chem. B, 2003, vol. 107, p. 3351.
Agmon, N., Chem. Phys. Lett., 1995, vol. 244, p. 453.
Agmon, N., J. Chem. Phys., 1996, vol. 93, p. 1714.
Schmitt, U.W. and Voth, G.A., J. Chem. Phys., 1999, vol. 111, p. 9361.
Schmitt, U.W. and Voth, G.A., J. Phys. Chem. B, 1998, vol. 102, p. 5547.
Vuilleumier, R. and Borgis, B., J. Chem. Phys., 1999, vol. 111, p. 4251.
Vuilleumier, R. and Borgis, B., Chem. Phys. Lett., 1998, vol. 284, p. 71.
Vuilleumier, R. and Borgis, B., J. Phys. Chem. B, 2003, vol. 107, p. 4261.
Kuznetsov, A.M., Charge Transfer in Physics, Chemistry and Biology, Reading: Gordon & Breach, 1995.
Kuznetsov, A.M. and Ulstrup, J., Electron Transfer in Chemistry and Biology: An Introduction to the Theory, Chichester: Wiley, 1999.
Walbran, S. and Kornyshev, A.A., J. Chem. Phys., 2001, vol. 114, p. 10039.
Von Grotthuss, C.J.D., Ann. Chim., 1806, vol. LVIII, p. 54.
Johnston, J., J. Am. Chem. Soc., 1909, vol. 31, p. 987.
Noyes, A., Z. Phys. Chem., 1910, vol. 70, p. 356.
Eucken, A., Z. Elektrochem., 1948, vol. 52, pp. 52, 255.
Gierer, A. and Wirtz, K., Ann. Phys., 1949, vol. 6, p. 17.
Ando, K. and Hynes, J.T., J. Phys. Chem. B, 1997, vol. 101, p. 10464.
Marx, D., Tuckerman, M.E., Hutter, J., and Parrinello, M., Nature, 1999, vol. 397, p. 601.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuznetsov, A.M., Ulstrup, J. Microscopic Models for Proton Transfer in Water and Strongly Hydrogen-Bonded Complexes with a Single-Well Proton Potential. Russian Journal of Electrochemistry 40, 1010–1018 (2004). https://doi.org/10.1023/B:RUEL.0000046484.21724.c0
Issue Date:
DOI: https://doi.org/10.1023/B:RUEL.0000046484.21724.c0