Abstract
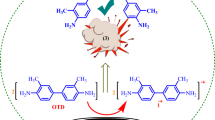
An analysis is performed and data are compared on the electrosynthesis of N-arylazoles and regularities of this process in conditions of a diaphragmless galvanostatic electrolysis (Pt, MeCN, Bu4NClO4) of a mixture of 1,4-dimethoxybenzene (DMB) with azoles (pyrazole, triazole, their derivatives, tetrazole). Electrolysis of an azole/DMB mixture leads to the formation of products of an ortho-substitution—1,4-dimethoxy-2-(azolyl-1)benzenes—and, simultaneously, hydrolytically unstable products of an ipso-bis-attachment—1,4-dimethoxy-1,4-di-(azolyl-1)cyclohexa-2,5-dienes. The overall yield of these compounds increases upon adding a base (collidine) or an acid (AcOH) into the initial mixture, and the basicity of initial azoles substantially affects the electrosynthesis results. New notions on the nature of nucleophilic species interacting with radical cation of DMB are considered. The species in question are complexes of azoles with one another or with collidine generated at the expense of the hydrogen bond, rather than azolate ions. Furthermore, the cathodic process is largely connected not with the generation of azolate ions (as a result of the reduction of initial azoles) but with the deprotonation of onium compounds (BH+)—products of the interaction of azoles or collidine with protons. The mechanism of electrosynthesis of N-arylazoles is discussed. The key stages of the synthesis are the attack of a nucleophile on the ipso- and, possibly, ortho-positions of the benzene ring of radical cation of DMB, as well as the rearrangement of the intermediate cation of 1,4-dimethoxy-1-(azolyl-1)arenonium into the cation of 1-(azolyl-1)-2,5-dimethoxyarenonium, which affects both the yield and ratio of final products of the reaction mixture.
Similar content being viewed by others
References
Petrosyan, V.A., Niyazymbetov, M.E., Pevzner, M.S., and Ugrak, B.I., Izv. Akad. Nauk SSSR, Ser. Khim., 1988, p. 1643.
Niyazymbetov, M.E., Mikhal'chenko, L.V., and Petrosyan, V.A., Abstracts of Papers, Vsesoyuz. konf. “Aromaticheskoe nukleofil'noe zameshchenie” (All-Union Conf. “Aromatic Nucleophilic Substitution”), Novosibirsk, 1989, p. 91.
Kai, Hu, Niyazymbetov, M.E., and Evans, D.H., Tetrahedorn Lett., 1995, vol. 36, p. 7027.
Catalan, J., Abbaud, J.L.M., and Elguero, J., Adv. Heterocyclic Chem., 1987, vol. 41, p. 250.
Bagal, L.I., Pevzner, M.S., Frolova, A.N., and Sheludyakova, N.I., Khim. Geterotsikl. Soedin., 1970, p. 259.
Huttel, R., Buchele, F., and Jochum, P., Chem. Ber., 1955, vol. 88, p. 1377.
Chauzov, V.A., Parchinskii, V.Z., Sinel'shchikova, E.V., and Petrosyan, V.A., Izv. Akad. Nauk, Ser. Khim., 2001, p. 1215.
Chauzov, V.A., Parchinskii, V.Z., Sinel'shchikova, E.V., Parfenov, N.N., and Petrosyan, V.A., Izv. Akad. Nauk, Ser. Khim., 2002, p. 917.
Chauzov, V.A., Parchinskii, V.Z., Sinel'shchikova, E.V., Burasov, A.V., Ugrak, B.I., Parfenov, N.N., and Petrosyan, V.A., Izv. Akad. Nauk, Ser. Khim., 2002, p. 1402.
Yoshida, K., Shigi, M., Kanbe, T., and Fueno, T., J. Org. Chem., 1975, vol. 40, p. 3805.
Laurent, A., Laurent, E., and Locher, P., Electrochim. Acta, 1975, vol. 20, p. 857.
Henton, D.R., McCreery, R.L., and Swenton, J.S., J. Am. Chem. Soc., 1980, vol. 45, p. 369.
Andreades, S. and Zahnow, E.W., J. Am. Chem. Soc., 1969, vol. 91, p. 4181.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Petrosyan, V.A. Electrosynthesis of N-Arylazoles by Electrolyzing a Mixture of Azole and 1,4-Dimethoxybenzene in a Diaphragmless Cell. Russian Journal of Electrochemistry 39, 1211–1220 (2003). https://doi.org/10.1023/B:RUEL.0000003448.35179.fa
Issue Date:
DOI: https://doi.org/10.1023/B:RUEL.0000003448.35179.fa