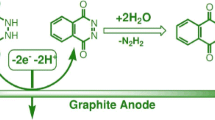
Abstract
Electrochemical reduction of phthalazines and 1,2,5-thiadiazoles containing nucleofugaceous groups at the carbon α-atoms are studied in aprotic and proton-donating media. Heteroatoms, substituents, and media are found to affect potentials and reaction path for the electroreduction. The factors determining the reductive opening of heterocycles are revealed. In diazines the heterocycle opening in annelated systems is induced by the electron transfer, provided there exist (a) a heteroatom–heteroatom bond and (b) an easily splitting-off group at the carbon α-atom, whose nucleofugacity is comparable with or exceeds that of the chloride ion. Stability of the 1,2,5-thiadiazole cycle toward its reduction is determined by the substituent and the medium nature. On adding a nucleofugaceous group to the molecule, the transfer of two electrons in an aprotic medium results in the heterocycle opening with the formation of iminonitrile; when two easily splitting-off groups are present, the electron transfer makes the cycle decompose into inorganic anions.
Similar content being viewed by others
References
Wawzonek, S. and Wagenknecht, J.H., J. Electrochem. Soc., 1963, vol. 110, p. 420.
Petrov, V.P. and Yakobson, G.G., Izv. Sib. Otd. Akad. Nauk SSSR, 1965, no. 7, p. 75.
Elson, C.M. and Liu, M.T.H., J. Chem. Soc., Chem. Commun., 1982, p. 415.
Yoon, S., Cho, J., and Kim, K., J. Chem. Soc., Perkin Trans. 1, 1998, p. 109.
Mitch, C.H., Brown, T.J., Bumaster, F.P., Calligaro, D.O., Dieckman, D., Merit, L., Peters, S.C., Quimby, S.J., Shannon, H.E., Shipley, L.A., Ward, J.S., Hansen, K., Oleser, P.H., Sauerberg, P., Seardown, M.J., Swedberg, M.J.B., Suzdak, P., and Greenwood, B., J. Med. Chem., 1997, vol. 40, p. 538.
Hanasaki, Y., Watanabe, H., Katsuura, K., Takayama, H., Shirakawa, S., Yamaguchi, K., Sakai, S., Ijichi, K., Fujiwara, M., Konno, K., Yokota, T., Shigeta, S., and Baba, M., J. Med. Chem., 1995, vol. 38, p. 2038.
Lund, H., The Chemistry of Carbon-Nitrogen Double Bond, Patai, S., Ed., New York: Interscience, 1970, p. 505.
Blok, N.I., Kachestvennyi khimicheskii analiz (Qualitative Chemical Analysis), Moscow: Goskhimizdat, 1952, p. 501.
Wiberg, K.B. and Lewis, T.P., J. Amer. Chem. Soc., 1970, vol. 92, p. 7154.
Lund, H. and Jensen, E.Th., Acta Chem. Scand., 1970, vol. 24, p. 1867.
Ivanova, V.Kh., Buzykin, B.I., and Bystrykh, N.N., Khim. Geterotsikl. Soedin., 1979, p. 541.
Buzykin, B.I., Yanilkin, V.V., Morozov, V.I., Maksimyuk, N.I., Eliseenkova, R.M., and Nastapova, N.V., Mendeleev Commun., 2000, no. 1, p. 34.
Yanilkin, V.V., Nastapova, N.V., Morozov, V.I., Eliseenkova, R.M., Maksimyuk, N.I., and Buzykin, B.I., in Elektrokhimicheskie, opticheskie i kineticheskie metody v khimii (Electrochemical, Optical, and Kinetic Methods in Chemistry), Kazan: Kazan. Univ., 2000, p. 356.
Yanilkin, V.V., Buzykin, B.I., Morozov, V.I., Nastapova, N.V., Maksimyuk, N.I., and Eliseenkova, R.M., Zh. Obshch. Khim., 2001, vol. 71, p. 1726.
Nastapova, N.V., Yanilkin, V.V., Morozov, V.I., Eliseenkova, R.M., Bredikhina, Z.A., Bredikhin, A.A., and Buzykin, B.I., Proc. Fifth Int. Manuel M. Baizer Symp. in Honor of Prof. J.M. Saveant, Workentin, M.S., Maran, F., and Chiba, K., Eds., The Electrochemical Society, Inc., 2002, vol. 10, p. 154.
Berdnikov, E.A., Fedorov, S.B., and Kargin, Yu.M., Zh. Obshch Khim., 1978, vol. 48, p. 875.
Buzykin, B.I., Bystrykh, N.N., Stolyarov, A.P., Zverev, V.V., and Kitaev, Yu.P., Khim. Geterotsikl. Soedin., 1976, p. 402.
Cook, J.C.S., Katrizkiy, A.R., Page, A.D., and Ramaian, M., J. Chem. Soc., Perkin Trans. 2, 1973, p. 1184.
Strunskaya, E.I., Yanilkin, V.V., Bredikhina, Z.A., Nastapova, N.V., Morozov, V.I., Maksimyuk, N.I., Sharafutdinova, D.R., and Bredikhin, A.A., Zh. Obshch. Khim., 2003, vol. 73, p. 852.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nastapova, N.V., Yanilkin, V.V., Eliseenkova, R.M. et al. Electroreduction of Phthalazines and 1,2,5-Thiadiazoles: Structural Factors Determining the Opening of Heterocycle. Russian Journal of Electrochemistry 39, 1166–1180 (2003). https://doi.org/10.1023/B:RUEL.0000003443.14644.6e
Issue Date:
DOI: https://doi.org/10.1023/B:RUEL.0000003443.14644.6e