Abstract
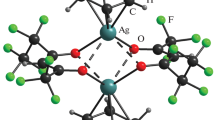
X-ray diffraction analysis of [Ag3(CHF2COO)3(H2O)2] revealed that its crystals are orthorhombic: space group Cmca, a = 13.809(4) Å, b = 15.975(2) Å, c = 12.244(2) Å, Z = 8. The thermogravimetric analysis showed that under the atmosphere of N2 and at 101.3 kPa, silver difluoroacetate melts at 488 K; the thermal decomposition reaction occurs in the interval 493–548 K with the formation of Ag. Under the mass-spectral experiment conditions at 521 K, two processes occur simultaneously, namely, evaporation and decomposition. The following ions were detected in the mass-spectrum of silver difluoroacetate: Ag2L+, Ag2R+, Ag2F+, Ag2O+, Ag2 +, Ag+, LH+, RCO+, R+ (L = CHF2COO, R = CHF2).
Similar content being viewed by others
REFERENCES
Sheldrick G.M., SHELXS97, SHELXL97, Göttingen (Germany): Univ. of Göttingen, 1997.
International Tables for X-Ray Crystallography, Ibers, J.A. and Hamilton, W.C., Eds., Birmingham: Kynoch Press, 1974. vol. 4, p. 278.
Coggon, P. and McPhail, A.T., J. Chem. Soc., Chem. Commun., 1972, no. 2, p. 91.
Eriksson, L. and Kritikos, M., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1995, vol. 51, p. 1508.
Karpova, E.V., Boltalin, A.I., Korenev, Yu.M., et al., Koord. Khim., 2000, vol. 26, no. 7, p. 489.
Karpova, E.V., Boltalin, A.I., Zakharov, M.A., et al., Z. Anorg. Allg. Chem., 1998, vol. 624, no. 4, p. 741.
Karpova, E.V., Boltalin, A.I., Korenev, Yu.M, and Troy-anov, S.I., Koord. Khim., 2000, vol. 26, no. 5, p. 384.
Smith, G., Reddy, A.N., Byriel, K.A., and Kennard, C.H.L., J. Chem. Soc., Dalton Trans., 1995, no. 22, p. 3565.
Wu, D.-D. and Mak, T.C.W., J. Chem. Soc., Dalton Trans., 1995, no. 16, p. 2671.
Smith, G., Reddy, A.N., Byriel, K.A., and Kennard, C.H.L., Polyhedron, 1994, vol. 13, p. 3135.
Karpova, E.V., Boltalin, A.I., Korenev, Yu.M., et al., Koord. Khim., 2001, vol. 27, no. 4, p. 311.
Paramonov, S.E., Mychlo, E.V., Troyanov, S.I., and Kuz'mina, N.P., Zh. Neorg. Khim., 2000, vol. 45, no. 12, p. 2003.
Karpova, E.V., Boltalin, A.I., Korenev, Yu.M., et al., Koord. Khim., 1999, vol. 25, no. 1, p. 70.
Karpova, E.V., Boltalin, A.I., Korenev, Yu.M., et al., Zh. Neorg. Khim., 1996, vol. 41, no. 7, p. 1185.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boltalin, A.I., Kas'yanov, S.A., Karpova, E.V. et al. Crystal Structure and Thermal Stability of Ag3(CHF2COO)3(H2O)2 . Russian Journal of Coordination Chemistry 30, 692–697 (2004). https://doi.org/10.1023/B:RUCO.0000043892.10551.fa
Issue Date:
DOI: https://doi.org/10.1023/B:RUCO.0000043892.10551.fa