Abstract
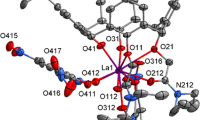
Complexones of a new class, viz., carboxy-functionalized calix[4]pyrrogallols, were synthesized. The per-O-(carboxymethyl)calix[4]pyrogallols obtained were established to exist in the (rel, cis, trans, trans)-configuration by 2D NMR spectroscopic data. According to the pH-potentiometric data, the interaction of these compounds with alkaline metal ions (Li+, Na+, K+, Cs+) and lanthanide ions (La3+, Gd3+, Lu3+) in a water—DMSO system produces 1 : 1 complexes. The specific features of complexation of per-O-(carboxymethyl)calix[4]pyrogallols, as compared to their acyclic analogs, with alkaline metal and lanthanide ions are due to the cooperative effect of donor groups preorganized on the calixarene matrix.
Similar content being viewed by others
References
G. R. Choppin, A. Dadgar, and E. N. Rizkalla, Inorg. Chem., 1986, 25, 3581.
K. Ohto, M. Yano, K. Inoue, T. Yamamoto, M. Goto, F. Nakashio, S. Shinkai, and T. Nagasaki, Anal. Sciences, 1995, 11, 893.
K. Ohto, M. Yano, K. Inoue, T. Yamamoto, T. Nagasaki, M. Goto, F. Nakashio, and S. Shinkai, Polyhedron, 1997, 16, 1655.
M. H. B. Grote Gansey, W. Verboom, F. C. J. M. van Veggel, V. Vetrogon, F. Arnaud-Neu, M.-J. Schwing-Weill, and D. N. Reinhoudt, Chem. Soc., Perkin Trans. 2, 1998, 2351.
W. Sliwa, T. Zujewska, and B. Bachowska, Pol. J. Chem., 2003, 77, 1079.
C. D. Gutsche, in Calixarenes 2001, Eds. Z. Asfari, V. Bohmer, and J. Vicens Harrowfield, Kluwer Academic Publishers, Dordrecht-Boston-London, 2001, 10.
H.-J. Shchneider, D. Guttes, and U. Schneider, J. Am. Chem. Soc., 1988, 110, 6449.
C. N. Pod'yachev, A. R. Mustafina, and W. D. Habicher, Izv. Akad. Nauk, Ser. Khim., 2003, 70 [Russ. Chem. Bull., Int. Ed., 2003, 52, 1].
A. W. Archer and P. A. Claret, J. Chem. Soc., Sect. C., 1970, 1296.
A. Giacosa, J. Praktisch. Chemie, 1879, 2, 396.
Technique of Organic Chemistry. V. VII. Organic Solvents. Physical Properties and Methods of Purification, Ed. A. Weissberger, Interscience Publisher, New York, 1955, 472 pp.
V. V. Aleksandrov, Kislotnost´ nevodnykh rastvorov [Acidity of Nonaqueous Solutions], Vishcha Shkola, Khar´kov, 1982, 159 pp. (in Russian).
O. Popovych, Anal. Chem., 1964, 36, 878.
Yu. I. Sal´nikov, A. N. Glebov, and F. V. Devyatov, Poliyadernye kompleksy v rastvorakh [Polynuclear Complexes in Solutions], Izd-vo KGU, Kazan, 1989, 287 pp. (in Russian).
L. Eberson, in The Chemistry of Carboxylic Acids and Esters, Ed. S. Patai, Interscience, New York, 1969, p. 272.
G. Arena, R.P. Bonomo, R. Cali, F. G. Gulino, G. G. Lombardo, D. Sciotto, R. Ungaro, and A. Casnati, Supra-molecular Chemistry, 1995, 4, 287.
T. Nagasaki, T. Arimura, and S. Shinkai, Bull. Chem. Soc. Jpn, 1991, 64, 2575.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pod"yachev, S.N., Mustafina, A.R., Koppehele, A.H. et al. Synthesis of per-O-(carboxymethyl)calix[4]pyrogallols and their complexation with some alkaline metal and lanthanide ions. Russian Chemical Bulletin 53, 1181–1188 (2004). https://doi.org/10.1023/B:RUCB.0000042271.85945.3d
Issue Date:
DOI: https://doi.org/10.1023/B:RUCB.0000042271.85945.3d