Abstract
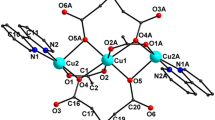
The reaction of tetrakis(trimethylsilyl)cyclobutadienylcyclopentadienyl cobalt complex (Me3Si)4C4CoCp with lithium metal in THF yielded the dilithium salt of cyclobutadiene dianion CBD2– stabilized by four trimethylsilyl groups, Li+ 2[(Me3Si)4C4]2–. The bridged CBD2– dianion was also synthesized by a similar procedure starting from the bridged cobalt complex, which was prepared from the reaction of 2,2,5,5,8,8,11,11-octamethyl-2,5,8,11-tetrasilacyclododeca-1,6-diyne with CpCo(CO)2 in refluxing octane. The aromaticity of the CBD2– is discussed on the basis of the structural characteristics and magnetic properties.
Similar content being viewed by others
References
(a) J. Thiele, Chem. Ber., 1900, 33, 666; (b) J. Thiele, Chem. Ber., 1901, 34, 68.
(a) K. Müllen, Chem. Rev., 1984, 84, 603; (b) W. N. Setzer and P. v. R. Schleyer, Adv. Organomet. Chem., 1985, 24, 353; (c) C. Schade and P. v. R. Schleyer, Adv. Organomet. Chem., 1987, 27, 169; (d) M. Rabinovitz, Top. Curr. Chem., 1988, 14, 99; (e) G. Maier, Angew. Chem., Int. Ed., 1988, 27, 309; (f) A. B. Sannigrahi, T. Kar, B. G. Niyogi, P. Hobza, and P. v. R. Schleyer, Chem. Rev., 1990, 90, 1061; (g) H. Bock, K. Ruppert, C. Näther, Z. Havlas, H. F. Herrmann, C. Arad, I. Göbel, A. John, J. Meuret, S. Nick, A. Rauschenbach, W. Seitz, T. Vaupel, and B. Solouki, Angew. Chem., Int. Ed. Engl., 1992, 31, 550; (h) A.-M. Sapse and P. v. R. Schleyer, in Lithium Chemistry: A Theoretical and Experimental Overview, Wiley, New York, 1995.
(a) G. Binsch, Naturwissenschaften, 1973, 60, 369; (b) T. Bally and S. Masamune, Tetrahedron, 1980, 36, 343; (c) P. Garratt, Aromaticity, Wiley, New York, 1986; (d) F. Toda and P. Garratt, Chem. Rev., 1992, 92, 1685; (e) V. L. Minkin, M. N. Glukhovtsev, and B. Y. Simkin, Aromaticity and Antiaroamticity, Wiley, New York, 1994; (f) G. Bertrand, Angew. Chem., Int. Ed., 1998, 37, 270.
Chem. Rev., Ed. P. v. R. Schleyer, 2001, 101, 1115.
J. S. McKennis, L. Brener, J. R. Schweiger, and R. Pettit, J. Chem. Soc., Chem. Commun., 1972, 365.
P. J. Garratt and R. Zahler, J. Am. Chem. Soc., 1978, 100, 7753.
(a) G. Boche, H. Etzrodt, M. Marsch, and W. Thiel, Angew. Chem., Int. Ed. Engl., 1982, 21, 133; (b) G. Boche and H. Etzrodt, Tetrahedron Lett., 1983, 24, 5477.
G. Boche, H. Etzrodt, M. Marsch, and W. Thiel, Angew. Chem., Int. Ed. Engl., 1982, 21, 133.
G. Boche, H. Etzrodt, W. Massa, and G. Baum, Angew. Chem., Int. Ed. Engl., 1985, 24, 863.
(a) T. Clark, D. Wilhelm, and P. v. R. Schleyer, Tetrahedron Lett., 1982, 23, 3547; (b) B. A. Hess, Jr., C. S. Ewig, and L. J. Schaad, J. Org. Chem., 1985, 50, 5869.
(a) A. J. Kos and P. v. R. Schleyer, J. Am. Chem. Soc., 1980, 102, 7928; (b) G. v. Zandwijk, R. A. J. Janssen, and H. M. Buck, J. Am. Chem. Soc., 1990, 112, 4155; (c) A. Skancke, Nouv. J. Chim., 1985, 9, 577; (d) M. Balci, M. L. McKee, and P. v. R. Schleyer, J. Phys. Chem., A, 2000, 104, 1246.
A. Sekiguchi, T. Matsuo, and H. Watanabe, J. Am. Chem. Soc., 2000, 122, 5652.
(a) H. Sakurai, Pure Appl. Chem., 1996, 68, 327; (c) A. Sekiguchi and T. Matsuo, J. Synth. Org. Chem. Jpn, 1999, 57, 945.
(a) A. Sekiguchi, T. Nakanishi, C. Kabuto, and H. Sakurai, J. Am. Chem. Soc., 1989, 111, 3748; (b) A. Sekiguchi, M. Ichinohe, C. Kabuto, and H. Sakurai, Organometallics, 1995, 14, 1092; (c) A. Sekiguchi, M. Ichinohe, M. Takahashi, C. Kabuto, and H. Sakurai, Angew. Chem., Int. Ed. Engl., 1997, 36, 1533; (d) A. Sekiguchi, K. Ebata, C. Kabuto, and H. Sakurai, J. Am. Chem. Soc., 1991, 113, 1464; (e) A. Sekiguchi, K. Ebata, C. Kabuto, and H. Sakurai, J. Am. Chem. Soc., 1991, 113, 7081; (f) K. Ebata, W. Setaka, T. Inoue, C. Kabuto, M. Kira, and H. Sakurai, J. Am. Chem. Soc., 1998, 120, 1335.
(a) A. Sekiguchi, T. Matsuo, and C. Kabuto, Angew. Chem., Int. Ed. Engl., 1997, 36, 2462; (b) A. Sekiguchi, T. Matsuo, and R. Akaba, Bull. Chem. Soc. Jpn, 1998, 71, 41; (c) T. Matsuo, H. Watanabe, M. Ichinohe, and A. Sekiguchi, Inorg. Chem. Commun., 1999, 2/10, 510; (d) T. Matsuo, H. Watanabe, and A. Sekiguchi, Bull. Chem. Soc. Jpn, 2000, 73, 1461; (e) T. Matsuo, H. Fure, and A. Sekiguchi, Chem. Commun., 1999, 1981; (f) T. Matsuo, H. Fure, and A. Sekiguchi, Bull. Chem. Soc. Jpn, 2000, 73, 2129.
(a) T. Matsuo, T. Mizue, and A. Sekiguchi, Chem. Lett., 2000, 896; (b) A. Sekiguchi, T. Matsuo, and M. Tanaka, Organometallics, 2002, 21, 1072.
A. Sekiguchi, M. Tanaka, T. Matsuo, and H. Watanabe, Angew. Chem., Int. Ed., 2001, 40, 1675.
(a) G. Maier, H. W. Lage, and H. P. Reisenauer, Angew. Chem., Int. Ed., 1981, 20, 976; (b) G. Maier, J. Neudert, and O. Wolf, Angew. Chem., Int. Ed., 2001, 40, 1674.
(a) H. Sakurai and J. Hayashi, J. Organomet. Chem., 1974, 70, 85; (b) J. R. Fritch, K. P. C. Vollhardt, M. R. Thompson, and V. W. Day, J. Am. Chem. Soc., 1979, 101, 2768.
(a) T. Kusumoto and T. Hiyama, Tetrahedron. Lett., 1987, 28, 18074; (b) W. Kaim and H. Bock, J. Organomet. Chem., 1979, 164, 218; (c) H. Sakurai, M. Kudo, K. Sakamoto, Y. Nakadaira, M. Kira, and A. Sekiguchi, Chem. Lett., 1988, 1441; (d) T. Matsuo, M. Tanaka, and A. Sekiguchi, Chem. Commun., 2001, 503.
R. Gleiter and M. Merger, Angew. Chem., Int. Ed. Engl., 1997, 36, 2426.
(a) H. Sakurai and J. Hayashi, J. Organomet. Chem., 1972, 39, 365; (b) C. Kabuto, J. Hayashi, H. Sakurai, and Y. Kitahara, J. Organomet. Chem., 1972, 43, C23.
K. Jonas and C. Krueger, Angew. Chem., Int. Ed. Engl., 1980, 19, 520.
C. Elschenbroich and A. Salzer, Organometallics, VCH Verlagsgesellschaft, Weingeim, 1989, p. 26.
(a) L. A. Paquette, W. Bauer, M. R. Sivik, M. Bühl, M. Feigel, and P. v. R. Schleyer, J. Am. Chem. Soc., 1990, 112, 8776; (b) H. Jiao and P. v. R. Schleyer, Angew. Chem., Int. Ed. Engl., 1993, 32, 1760.
K. Ishii, N. Kobayashi, T. Matsuo, M. Tanaka, and A. Sekiguchi, J. Am. Chem. Soc., 2001, 123, 5356.
C. Lambert and P. v. R. Schleyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 1129.
E. Kloster-Jensen and G. A. Eliassen, Angew. Chem., Int. Ed. Engl., 1985, 24, 565.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sekiguchi, A., Matsuo, T., Tanaka, M. et al. Tetrasilyl-substituted cyclobutadiene dianion dilithium salt: synthesis and structure. Russian Chemical Bulletin 53, 1109–1115 (2004). https://doi.org/10.1023/B:RUCB.0000041308.25889.77
Issue Date:
DOI: https://doi.org/10.1023/B:RUCB.0000041308.25889.77