Abstract
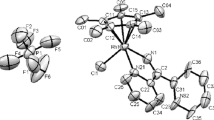
The room temperature reaction of the complex cis,trans,cis-[Ir(H)2(PPh3)2(Solv)2]PF6 (Solv is a solvent) with the imine PhCH2N=CHPh in acetone generates (with loss of H2) the orthometallated complex [Ir(H){PhCH2N=CH(o-C6H4)}(PPh3)2(Me2CO)]PF6 (3) containing a five-membered cyclometallated imine moiety. In MeOH, the reaction at an imine : Ir ratio = 1 leads to the corresponding MeOH analog of 3, while with excess imine, the mixed orthometallated imine/bezylamine complex [Ir(H){PhCH2N=CH(o-C6H4)}(PPh3)2(PhCH2NH2)]PF2 (4) is formed; the source of the coordinated amine is an Ir-promoted hydrolysis of the imine, the water likely coming from imine. Complexes 3 and 4 are fully characterized by elemental analysis, 1H and 31P{1H} NMR spectroscopy, and X-ray crystal structure analysis.
Similar content being viewed by others
References
R. R. Schrock and J. A. Osborn, J. Am. Chem. Soc., 1971, 93, 2397.
L. M. Haines and E. Singleton, J. Chem. Soc., Dalton Trans., 1972, 1891.
R. R. Schrock and J. A. Osborn, J. Am. Chem. Soc., 1976, 98, 4450, and the references therein.
R. H. Crabtree, H. Felkin, and G. E. Morris, J. Organomet. Chem., 1977, 141, 205.
R. R. Schrock and J. A. Osborn, J. Chem. Soc., Chem. Commun., 1970, 567.
(a) A. Levi, G. Modena, and G. Scorrano, J. Chem. Soc., Chem. Commun., 1975, 6; (b) C. J. Longley, T. J. Goodwin, and G. Wilkinson, Polyhedron, 1986, 5, 1625.
J. R. Shapley, R. R. Schrock, and J. A. Osborn, J. Am. Chem. Soc., 1969, 91, 2816.
R. H. Crabtree, P. C. Demou, D. Eden, J. M. Mihelcic, C. A. Parnell, J. M. Quirk, and G. E. Morris, J. Am. Chem. Soc., 1982, 104, 6994.
P. Marcazzan, M. B. Ezhova, B. O. Patrick, and B. R. James, C. R. Chimie, 2002, 5, J. Osborn issue, 373.
B. R. James, Catalysis Today, 1997, 37, 209.
P. Marcazzan, B. O. Patrick, and B. R. James, Organometallics, 2003, 22, 1177.
(a) G.-J. Kang, W. R. Cullen, M. D. Fryzuk, B. R. James, and J. P. Kutney, J. Chem. Soc., Chem. Commun., 1988, 1466; (b) W. R. Cullen, M. D. Fryzuk, B. R. James, J. P. Kutney, G.-J. Kang, G. Herb, I. S. Thorburn, and R. Spogliarich, J. Mol. Catal., 1990, 62 243; (c) A. G. Becalski, W. R. Cullen, M. D. Fryzuk, B. R. James, G. J. Kang, and S. J. Rettig, Inorg. Chem., 1991, 30, 5002.
M. D. Fryzuk and W. E. Piers, Organometallics, 1990 9, 986
P. Marcazzan, Ph. D. Thesis, The University of British Columbia, Vancouver, 2002.
R. H. Crabtree, G. G. Hlatky, C. P. Parnell, B. E. Segmüller, and R. J. Uriarte, Inorg. Chem., 1984, 23, 354.
K. Gruet, R. H. Crabtree, D. H. Lee, L. Liable-Sands, and A. L. Rheingold, Organometallics, 2000, 19, 2228.
M. Crespo, M. Martinez, J. Sales, X. Solans, and M. Font-Bardía, Organometallics, 1992, 11, 1288.
(a) J. F. van Baar, K. Vrieze, and D. J. Stufkens, J. Organomet. Chem., 1975, 85, 249; (b) J. F. van Baar, K. Vrieze, and D. J. Stufkens, J. Organomet. Chem., 1975, 97, 461.
M. Calligaris and L. Randaccio, in Comprehensive Coordination Chemistry, Eds. G. Wilkinson, R. D. Gillard, and J. A. McCleverty, Pergamon Press, Oxford, 1987, 2, 715.
H. D. Kaesz and R. B. Saillant, Chem. Rev., 1972, 72, 231.
I. Omae, Chem. Rev., 1979, 79, 287.
M. B. Ezhova, B. O. Patrick, B. R. James, M. E. Ford, and B. F. J. Waller, Izv. Akad. Nauk, Ser. Khim., 2003, 2562 [Russ. Chem. Bull., Int. Ed., 2003, 52, No. 12].
D. D. Perrin, W. L. F. Amarego, and D. R. Perrin, Purification of Laboratory Chemicals, Pergamon Press, Oxford, 1980.
J. E. Huheey, E. A. Keiter, and R. L. Keiter, Inorganic Chemistry, Harper Collins, New York, 1993, p. 370.
D. Carmona, J. Ferrer, M. Lorenzo, M. Santander, S. Ponz, F. J. Lahoz, and L. A. Oro, Chem. Commun., 2002, 870.
D.-H. Lee, J. Chen, J. W. Faller, and R. H. Crabtree, Chem. Commun., 2001, 213.
S. Grundemann, A. Kovacevic, M. Albrecht, J. W. Faller, and R. H. Crabtree, Chem. Commun., 2001, 2274.
D. E. Fogg and B. R. James, Inorg. Chem., 1995, 34, 2557.
R. H. Crabtree and G. E. Morris, J. Organomet. Chem., 1977, 135, 395.
d*TREK: Area Detector Software. Version 4.4, Molecular Structure Corporation, The Woodlands, TX 77381, USA 1996–1998.
A. Altomare, M. C. Burla, G. Cammalli, M. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori, and A. Spagna, SIR97, J. Appl. Cryst., 1999, 32, 115.
P. T. Beurskens, G. Admiraal, G. Beurskens, W. P. Bosman, R. de Gelder, R. Israel, and J. M. M. Smits, The DIRDIF-94 Program System, Technical Report of the Crystallography Laboratory, University of Nijmegen, The Netherlands, 1994.
D. T. Cromer and J. T. Waber, International Tables for X-Ray Crystallography, The Kynoch Press, Birmingham, UK, 1974, 4, Table 2.2 A.
J. A. Ibers and W. C. Hamilton, Acta Crystallogr., 1964, 17, 781.
D. C. Creagh and W. J. McAuley, International Tables for X-Ray Crystallography, Ed. A. J. C. Wilson, Kluwer Academic Publishers, Boston, 1992, C, 219, Table 4.2.6.8.
D. C. Creagh and J. H. Hubbell, International Tables for X-Ray Crystallography, Ed. A. J. C. Wilson, Kluwer Academic Publishers, Boston, 1992, C, 200, Table 4.2.4.3.
teXsan: Crystal Structure Analysis Package, Molecular Structure Corporation, The Woodlands, TX 77381, USA, (1996–1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marcazzan, P., Patrick, B.O. & James, B.R. Iridium(iii)-(hydrido)cyclometallated-imine complexes and metal-promoted hydrolytic cleavage of imines. Russian Chemical Bulletin 52, 2715–2721 (2003). https://doi.org/10.1023/B:RUCB.0000019891.73897.e4
Issue Date:
DOI: https://doi.org/10.1023/B:RUCB.0000019891.73897.e4