Abstract
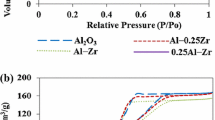
The characteristics of Al2O3, ZrO2 and three binary mixtures of ZrO2-Al2O3 were studied by determining their BET surface areas, micropore surface area, total pore volume, adsorption-desorption isotherms, the X-ray diffractogram, surface acidity and catalytic functionality for cumene cracking. The XRD results show that the incorporation of alumina into the zirconia from 50% and beyond renders it amorphous. Furthermore, the mixed oxide containing 50% alumina and 50% zirconia had the highest BET surface area of 199.9 m2/g whilst pure zirconia had the lowest BET surface area of 37.19 m2/g. The pores for all the mixed oxides were found to be monomodal and zirconia pores were more open. The results of the acidity measurements and cumene cracking functionality indicates that whilst pure zirconia has low total acidity, the incorporation of alumina increases its acidity through a synergistic effect.
Similar content being viewed by others
REFERENCES
H. Topsoe, B.S. Clausen, F.E. Massoth: Hydrotreating Catalysis Science and Technology.Vol. 11. Springer-Verlag, Berlin, Heidelberg, New York 1996.
B.C. Gates, J.R. Katzer, G.C.A. Schuit: Chemistry of Catalytic Processes,p. 396. MacGraw-Hill, 1979.
D.D. Whitehurst, T. Isoda, I. Mochida: Adv. Catal., 42,345 (1998).
J. Ramirez, M. Vrinat, S. Fuentes, G. Diaz, M. Breysse, M. Lacroix: Appl. Catal., 53, 211 (1989).
US Patent No. 76-690254, Exxon Res. and Eng. Comp., (1976).
S.K. Maity, M.S. Rana, B.N. Srinivas, S.K. Bej. G. Murali Dhar, T.S.R. Prasada Rao: J. Mol. Catal., 153, 121 (2000).
R.J. Bertolarini, T.A. Sue-A-Quan: Chem. Abstr., 90, 189614 (1979).
S. Kurokawa, T. Miyasaki: Chem. Abstr., 84, 47020 (1976).
H. Shimada, T. Sato, Y. Yoshimura, J. Hiraishi, A. Nishijima: J. Catal., 110, 275 (1988).
T. Klimova, D.S. Casodos, I. Ramirez: Catal. Today, 43, 135 (1998).
K.C. Pratt, J.V. Sanders, V. Christov: J. Catal., 124, 416 (1990).
C.T. Fishel, R.T. Davis: Catal. Lett., 25, 87 (1994).
M. Vrinat, M. Breysse, C. Geantet, J. Ramirez, F. Massoth: Catal. Lett., 26,25 (1994).
M. Vrinat, D. Hamon, M. Breysse, B. Durand, T. des Courières: Catal. Today, 20, 273 (1994).
F.F. Lange, D.J. Green: Adv. Ceramics, p. 217. Elsevier, Amsterdam (1983).
M.S. Rana: Ph.D Thesis. H.N.B. Garhwal University, Srinagar (U.P) India, (1999).
J.A. Noh, J.A. Schwartz: J. Colloid Interface Sci., 27 ,531 (1986).
T. Klinova, M.L. Rojas, P. Castillo, R. Caevas, J. Ramirez: Micro. Meso. Mater., 20,293 (1998).
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemiewska: Pure Appl. Chem., 57, 603 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aberuagba, F., Kumar, M., Gupta, J.K. et al. Characterization of ZrO2- Al2O3mixed oxides support prepared by urea hydrolysis. Reaction Kinetics and Catalysis Letters 80, 311–317 (2003). https://doi.org/10.1023/B:REAC.0000006140.54392.e3
Issue Date:
DOI: https://doi.org/10.1023/B:REAC.0000006140.54392.e3