Abstract
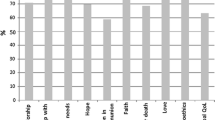
Mistletoe preparations standardised to the content of mistletoe lectin are supposed to improve quality of life (QoL) in patients with cancer. To obtain a validated and sensitive research instrument, the Life Quality Lectin-53 (LQL-53) Questionnaire was developed in three phases: item generation via interviews with 42 patients, item selection by means of a study with 109 cancer patients, and psychometric testing. The LQL-53 includes 46 items assigned to the subscales ‘General well-being’, ‘Emotional well-being’, ‘Vitality’ ‘Hope’, ‘Locus of control’, Social relationships', and ‘Physical complaints’, plus seven items dealing with possible adverse effects of mistletoe treatment. Psychometric testing was carried out in a study with 112 patients with solid tumours who were treated with a mistletoe preparation standardised to mistletoe lectins for 12 weeks. Internal consistency (Cronbach's α ) was between 0.72 and 0.94. Test–retest reliability was ≥r of 0.72. Subscales correlated highly with external criteria. Construct validity, as determined by Multitrait Scaling Analysis, resulted in an optimal assignment of items to subscales (scaling success) for four of the subscales. During the course of the therapy, significant improvement in QoL was found in all subscales. In two of the subscales, effect size was high (>0.80), and in five other subscales it varied between 0.53 and 0.78.
Similar content being viewed by others
References
Antonovsky A. Gesundheitsforschung versus Krankheitsforschung. In: Franke A, Brode M (eds), Psychosomatische Gesundheit. Versuch einer Abkehr vom Pathogenesekonzept. Tübingen: Verlag Deutsche Gesellschaft für Verhaltenforschung, 1993.
Bartsch HH, Bengel J (eds). Salutogenese in der Onkologie. Basel: Karger, 1997.
Pandey M, Singh SP, Behere PB, et al. Quality of life in patients with early and advanced carcinoma of the breast. Eur J Surg Oncol 2000; 26(1): 20-24.
Hürny C, Bernhard J, Coates AS. Impact of adjuvant therapy on quality of life in women with node-positive operable breast cancer. International Breast Cancer Study Group. Lancet 1996; 347(9011): 1279-1284.
Weis J, Bartsch HH, Hennies F, et al. Complementary medicine in cancer patients. Demand, patients' attitudes and psychological beliefs. Onkologie 1998; 21: 144-149.
Heiny B-M, Albrecht V, Beuth J. Lebensqualitätsstabilisierung durch mistellektin-I-normierten Extrakt beim fortgeschrittenen kolorektalen Karzinom [Stabilization of quality of life of patients with advanced colorectal carcinoma through standardized mistletoe-lectin-I extract]. Onkologe 1998; 4: 35-39.
Hager ED, Kleef R, Popa C, et al. Komplementärtherapie des nicht kurativ resektablen Pankreaskarzinoms [Complementary treatment of the non-curativ resectable pancreatic carcinoma]. Dtsch ZschrOnkol 1995; 27: 115-121.
Friess H, Beger HG, Kunz J, Funk N, Schilling M, Büchler MW. Treatment of advanced pancreatic cancer with mistletoe: Results of a pilot trial. Anticancer Res 1996; 16: 915-920.
Wolf P, Freudenberg N, Konitzer M. Analgetische und stimmungsaufhellende Wirkung bei Malignom-Patienten unter hochdosierter Viscum album-Infusionstherapie (Vysorel). Dtsch Zschr Onkol 1994; 26: 52-54.
Heiny BM Additive Therapie mit standardisiertem Mistelextrakt reduziert die Leukopenie und verbessert die Lebensqualität von Patientinnen mit fortgeschrittenem Mammakarzinom unter palliativer Chemotherapie (VEC-Schema) [Additional therapy with standardized mistletoe extract reduces leukopenia and improves quality of life of patients with advanced breast cancer receiving palliative chemotherapy (VEC-scheme)]. Cancer Res 1991; 12: 36-45.
Kiene H. Klinische Studien zur Misteltherapie karzinomatöser Erkrankungen. Erfahrungsheilkunde 1991; 3a: 222-227.
Kleijnen J, Knipschild P. Mistletoe treatment for cancer. Review of controlled trials in humans. Phytomedicine 1994; 1: 255-260.
Hauser SP. Klinische Anwendung von Mistelpräparaten in der Onkologie. Mistel — Wunderkraut oder Medikament? Therapiewoche 1993; 43/3: 76-81.
Resch KL, Windeler J. Studies in mistletoe therapy and cancer: Still waiting for the big step forward. Res Complementary Natural Class Med 2001; 8(4): 236-238.
Wetzel D, Schäfer M. Results of a randomised, placebo-controlled multicentre study with PS76A2 (standardised mistletoe preparation) in patients with breast cancer receiving adjuvant chemotherapy. Phytomedicine 2000; 7(Suppl. II): 34.
Hürny Ch, Heusser P, Bernhard J, Castiglione M, Cerny Th. Verbessern nicht konventionelle Zusatztherapien die Lebensqualität von Krebspatienten? [Do non-conventional complementary therapies improve the quality of life of cancer patients?] Schweiz Med Wochenschr 1994; 142: 55-63.
Boie D, Gutsch J. Helixor bei Kolon-und Rektumkarzinom [Helixor for colon and rectum carcinoma]. In: Denck H, Karrer K (eds), Kolorektale Tumoren. Heidelberg: Fischer Verlag; Schriftenreihe Krebsgeschehen 1980; 23: 65-76.
Buck G. Viscum album in der Krebstherapie [Viscum album in cancer treatment]. Z AIIg Med 1996; 72: 359-362.
Shumaker S, Anderson RT, Czaijkowski SM. Psychological tests and scales. In: Spilker B (ed.), Quality of Life Assessment in Clinical Trial. New York: Raven; 1990: 95-113.
Stoll BA. Can unorthodox cancer therapy improve quality-of-life? Ann Oncol 1993; 4: 121-123.
Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia: Lippincott-Raven, 1996.
Keller SD, Ware JE, Gandek B, et al. Testing the equivalence of translations of widely used response choice labels: Results from the IQOLA project. J Clin Epidemiol 1998; 51(11): 933-944.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Nat Cancer Inst 1993; 85: 365-376.
Von Steinbüchel N, Bullinger M, Kirchberger I. The Munich Life Dimension List (MLDL): Development and testing of an instrument for the generic assessment of quality of life. Zschr Med Psychol 1999; 8: 99-112.
McNair DM, Lorr M, Droppelman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service, 1971.
Ferring D, Filipp SH. Der Fragebogen zur Erfassung gesundheitsbezogener Kontrollüberzeugungen (FEGK) [The questionnaire for the assessment of health-related locus of control (FEGK)]. Z Klin Psychol 1989; 18(3): 285-289.
Krampen G. Skalen zur Erfassung von Hoffnungslosigkeit (H-Skalen). Handanweisung [Scales for the Assessment of Hopelessness (H-Scales). Manual]. Göttingen: Hogrefe, 1994.
Nunnally JC, Bernstein IR. Psychometric Theory. 3rd ed. New York: McGraw-Hill, 1994.
Tulsky DS. An introduction to test theory. Oncology 1990; 4(5): 43-48.
Hays RD, Hayashi T, Carson S, Ware JE. User's Guide for the Multitrait Analysis Program (MAP). Santa Monica: Rand Corporation, 1988.
Ware JE, Gandek B for the IQOLA Project Group. Methods for testing data quality, scaling assumptions, and reliability: The IQOLA project approach. J Clin Epidemiol 1998; 51(11): 945-952.
Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull 1959; 56(2): 81-105.
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988.
Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in 1989; 3: 178-189.
Jackson JL, Chamberlin J, Kroenke K. Predictors of patient satisfaction. Soc Sci Med 2001; 4: 609-620.
Fitzpatrick R. Surveys of patient satisfaction: I — important general considerations. Br Med J 1991; 302: 887-889.
Williams SA, Swanson MS. The effect of reading ability and response formats on patients' abilities to respond to a patient satisfaction scale. J Contin Educ Nurs 2001; 32: 60-67.
Hays RD, Ware JE. Social desirability and patient satisfaction ratings. Med Care 1986; 24: 519-524.
Finger T, Kirchberger I, Dietze S, Schäfer M. Development of a questionnaire to assess the effects of mistletoe treatment on the quality of life of cancer patients. Poster presented at the 5th, Annual Conference of the International Society for Quality of Life Research, Baltimore, 15–17 November 1998.
Kirchberger I. Anwendungsbeobachtung mit Lektinol® zur Validierung eines Fragebogens zur Erfassung des Nutzens einer Therapie mit einem Mistelextrakt bei Patienten mit soliden Tumoren. [Prospective cohort study on Lektinol® to validate a questionnaire for the assessment of the benefit of a therapy with mistletoe extract in patients with solid tumors]. Integrated Clinical Trial Report, Köln, Madaus AG, 2000.
Grossarth-Maticek R, Kiene H, Baumgartner SM, Ziegler R. Use of Iscador, an extract of European mistletoe (Viscum album), in cancer treatment: Prospective nonrandomized and randomized matched-pair studies nested within a cohort study. Altern Therap Health Medi 2001; 7: 57-76.
Scientific Advisory Committee of the Medical Outcomes Trust. Assessing health status and quality-of-life instruments: Attributes and review criteria. Qual Life Res 2002; 11(3): 193-205.
Sigurdardottir V, Bolund C, Brandberg Y, Sullivan M. The impact of generalized malignant melanoma on quality of life evaluated by the EORTC questionnaire technique. Qual Life Res 1993; 2: 193-203.
Ringdal Gl, Ringdal K. Testing the EORTC quality of life questionnaire on cancer patients with heterogeneous diagnoses. Qual Life Res 1993; 2: 129-140.
Aaronson NK, Ahmedzai S, Bullinger M, et al. The EORTC core quality of life questionnaire: Interim results of an international field study. In: Osoba D (ed.), Effect of Cancer on Quality of Life. Boca Raton, FL: CRC, 1991: 185-203.
Aaronson NK, Cull A, Kaasa S, Sprangers MAG, EORTC Study Group on Quality of Life. The European Organisation for Research and Treatment of Cancer (EORTC) modular approach to quality of life assessment in oncology: an update. In: Spilker B (ed.), Quality of Life and Pharmacoeconomics in Clinical Trials. New York: Raven Press, 1996.
Coates A, Glasziou P, McNeil D. On the receiving end — III Measurement of quality of life during cancer chemotherapy. Ann Oncol 1990; 1: 213-217.
Steuer-Vogt MK, Bonkowsky V, Ambrosch P, et al. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: A randomised controlled clinical trial. Eur J Cancer 2001; 37: 23-31.
Osoba D, Zee B, Pater J, Warr D, Kaizer L, Latreille J. Psychometric properties and responsiveness of the EORTC Quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual Life Res 1994; 3: 353-364.
King T. The interpretation of scores from the EORTC quality of life questionnaire QIQ-C30. Qual Life Res 1996; 5: 555-567.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kirchberger, I., Wetzel, D. & Finger, T. Development and validation of an instrument to measure the effects of a mistletoe preparation on quality of life of cancer patients: The Life Quality Lectin-53 (LQL-53) Questionnaire. Qual Life Res 13, 463–479 (2004). https://doi.org/10.1023/B:QURE.0000018481.33943.97
Issue Date:
DOI: https://doi.org/10.1023/B:QURE.0000018481.33943.97