Abstract
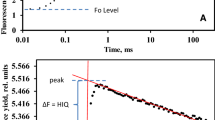
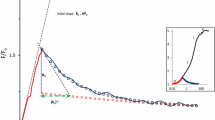
The low-wave phenomenon, i.e., the transient drop of yield of modulated chlorophyll fluorescence shortly after application of a pulse of saturating light, was investigated in intact leaves of tobacco and Camellia by measuring fluorescence, CO2 assimilation and absorption at 830 nm simultaneously. Limitations on linear electron flow, due to low electron acceptor levels that were induced by low CO2, induced the low waves of chlorophyll fluorescence. Low-wave amplitudes obtained under different CO2 concentrations and photon-flux densities yielded single-peak curves when plotted as functions of fluorescence parameters such as ΦPS II (quantum yield of Photosystem II) and qN (coefficient of non-photochemical quenching), suggesting that low-wave formation depends on the redox state of the electron transport chain. Low waves paralleled redox changes of P700, the reaction center of Photosystem I (PS I), and an additional electron flow through PS I was detected during the application of saturating pulses that induced low-waves. It is suggested that low waves of chlorophyll fluorescence are induced by increased non-photochemical quenching, as a result of the formation of a trans-thylakoid proton gradient due to cyclic electron flow around PS I.
Similar content being viewed by others
References
Arnon DI and Chain RK (1975) Regulation of ferredoxin-catalysed photosynthetic phosphorylation. Proc Natl Acad Sci USA 72: 4961–4965
Bendall DS and Manasse RS (1995) Cyclic photophosphorylation and electron transport. Biochim Biophys Acta 1229: 23–38
Bilger W, Heber U and Schreiber U (1988) Kinetic relationships between energy-dependent fluorescence quenching, light scattering, chlorophyll luminescence and proton pumping in intact leaves. Z Naturforsch 43c: 877–887
Fork DC and Herbert SK (1993) Electron transport and photophosphorylation by Photosystem I in vivo in plants and cyanobacteria. Photosynth Res 36: 149–168
Genty B, Briantais JM and Baker N (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92
Hagen C, Bornman JF and Braune W (1992) Reversible lowering of modulated chlorophyll fluorescence after saturating flashes in Haematococcus lacustris (Volvocales) at room temperature. Physiol Plant 86: 593–599
Harbinson J and Foyer CH (1991) Relationships between the efficiencies of Photosystem I and II and stromal redox state in CO2-free air. Evidence for cyclic electron flow in vivo. Plant Physiol 97: 41–49
Heber U, Egneus H, Hanck U, Jensen M and Koster S (1978) Regulation of photosynthetic electron transport and photophosphorylation in intact chloroplasts and leaves of Spinacia oleracea L. Planta 143: 41–49
Heber U, Neimanis S, Siebke K, Schonknecht G and Katona E (1992) Chloroplast energization and oxidation of P700/plastocyanin in illuminated leaves at reduced levels of CO2 or oxygen. Photosynth Res 34: 433–447
Heber U, Gerst U, Krieger A, Neimanis S and Kobayashi Y (1995) Coupled cyclic electron transport in intact chloroplasts and leaves of C3 plants: does it exist? if so, what is its function? Photosynth Res 46: 269–275
Heber U, Bligny R, Streb P and Douce R (1996) Photorespiration is essential for the protection of the photosynthetic apparatus of C3 plants against photoinactivation under sunlight. Bot Acta 109: 307–315
Heber U, Bilger W, Bligny R and Lange OL (2000) Phototolerance of lichens, mosses and higher plants in an alpine environment: analysis of photoreactions. Planta 211: 770–780
Joët T, Cournac L, Horvath EM, Medgyesy P and Peltier G (2001) Increased sensitivity of photosynthesis to antimycin A induced by inactivation of the chloroplast ndhB gene. Evidence for a participation of the NADH-dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol 125: 1919–1929
Juhler RK, Miller M, Simpson D and Cox RP (1993) Chlorophyll fluorescence transients in a barley mutant lacking Photosystem I. Photosynth Res 35: 305–310
Katona E, Neimanis S, Schonknecht G and Heber U (1992) Photosystem I-dependent cyclic electron transport is important in controlling Photosystem II activity in leaves under conditions of water stress. Photosynth Res 34: 449–464
Klimov VV, Shuvalov VA and Heber U (1985) Photoreduction of pheophytin as a result of electron donation from the water-splitting system to Photosystem-II reaction centers. Biochim Biophys Acta 809: 345–350
Klughammer C and Schreiber U (1991) Analysis of light-induced absorbance changes in the near-infrared spectral region. I. Characterization of various components in isolated chloroplasts. Z Naturforsch 46c: 233–244
Klughammer C and Schreiber U (1994) An improved method, using saturating light pulses, for the determination of Photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192: 261–268
Kobayashi Y, Neimanis S and Heber U (1995) Coupling ratios H+/e=3 versus H+/e=2 in chloroplasts and quantum requirements of net oxygen exchange during the reduction of nitrate, ferricyanide or methylviologen. Plant Cell Physiol 36: 1613–1620
Krause H and Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349
Larcher W and Neuner G (1989) Cold-induced sudden reversible lowering of in vivo chlorophyll fluorescence after saturating light pulses. A sensitive marker for chilling susceptibility. Plant Physiol 89: 740–742
Makino A, Miyake C and Yokota A (2002) Physiological functions of the water-water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol 43: 1017–1026
Maxwell K and Johnson GN (2000) Chlorophyll fluorescence — a practical guide. J Exp Bot 51: 659–668
Öquist G and Chow WS (1992) On the relationship between the quantum yield of Photosystem II electron transport, as determined by chlorophyll fluorescence and the quantum yield of CO2-dependent O2 evolution. Photosynth Res 33: 51–62
Peterson RB (1990) Effects of irradiance on the in vivo CO2:O2 specificity factor in tobacco using simultaneous gas exchange and fluorescence techniques. Plant Physiol 94: 892–898
Schreiber U and Neubauer C (1990) O2-dependent electron flow, membrane energization and the mechanism of non-photochemical quenching of chlorophyll fluorescence. Photosynth Res 25: 279–293
Schreiber U, Schliwa U and Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62
Schreiber U, Klughammer C and Neubauer C (1988) Measuring P700 absorbance changes around 830 nm with a new type of pulse modulated system. Z Naturforsch 43: 686–698
Takahama U, Shimizu-Takahama M and Heber U (1981) The redox state of the NADP system in illuminated chloroplasts. Biochim Biophys Acta 637: 530–539
Tsuyama M, Shibata M and Kobayashi Y (2003) Leaf factors affecting the relationship between chlorophyll fluorescence and the rate of photosynthetic electron transport as determined from CO2 uptake. J Plant Physiol 160: 1131–1139
von Caemmerer S and Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387
van Kooten O and Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150
Xyländer M and Hagen C (2002) 'Low-waves' in chlorophyll fluorescence kinetics indicate deprivation of bicarbonate. Photosynth Res 72: 255–262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsuyama, M., Shibata, M., Kawazu, T. et al. An Analysis of the Mechanism of the Low-wave Phenomenon of Chlorophyll Fluorescence. Photosynthesis Research 81, 67–76 (2004). https://doi.org/10.1023/B:PRES.0000028394.60328.b5
Issue Date:
DOI: https://doi.org/10.1023/B:PRES.0000028394.60328.b5