Abstract
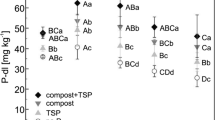
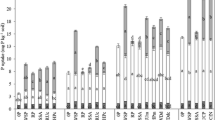
Calcareous dark brown red soil (calcixerollic xerochrept) from northern Syria was used in a pot experiment to study the fate of triple super phosphate fertilizer (TSP) with and without a crop (local durum wheat [Triticum turgidum L. group durum (Desf.)] cv. Bohouth). The soil received 17 μg P/g soil of 32P-labeled TSP, and samples were collected from soils and plants at successive dates. Soil inorganic P was ≈ 94% of total soil P, with only 50–80% being soluble. Calcium phosphate compounds were the dominant fraction (≤ 68%) of the soluble inorganic soil P followed by occluded iron phosphate (≤ 48%), and all other fractions were ≤ 9%. Isotopic measurements showed that ≈ 50% of fertilizer P was non–exchangeable within 2 days, and TSP values in each fraction of soil inorganic P fluctuated in relatively similar proportions to the concentrations of P fractions in soil. Available P (soil & TSP) in cropped soil was more than that in the uncropped soil, and plants had no effect on the distribution of P from fertilizer amongst the different soil P fractions.
Similar content being viewed by others
References
Adams M A and Pate J S 1992 Availability of inorganic and organic forms of phosphorus to lupinus (Lupinus spp.). Plant Soil 145, 107–113.
Akinremi O O and Cho C M 1991 Phosphate and accompanying cation transport in a calcareous cation exchange resin system. Soil Sci. Soc. Am. J. 55, 959–964.
Al-Merey R, Al-Hameish M and Asfary A F 1999 A new method of phosphorus determination with an improved pretreatment of occluded iron phosphate fraction in soils. Commun. Soil Sci. Plant Anal. 30, 2419–2428.
Barber S A 1980 Soil plant interaction in the phosphate nutrition of plant. In The Role of Phosphorus in Agriculture. Eds. F E Khasaweneh et al. pp. 591–615. American Soc. Agron., Madison, WI.
Barber S A 1984 Soil nutrient bioavailability. pp. 9–51. John Wiley and Sons, New York.
Brar S P S and Cox F R 1991 Phosphorus sorption and availability indexes as effected by properties of calcareous soils. Commun. Soil Sci. Plant Anal. 22, 1225–1241.
Chang S C and Jackson M L 1957 Fractionation of Soil Phosphorus. Soil Sci. 84,133–144.
Fardeau J C 1996 Dynamics of phosphate in soils. An isotopic outlook. Fert. Res. 45. 91–100.
Fixen P E and Grove J H 1990 Testing soil for phosphorus. In Soil Testing and Plant Analysis. 3d edn. pp. 141–180. Soil Sci. Soc. Am. Madison, WI.
Ghonsikar C P and Musande V G 1978 Madras Agri. J. 65, 796–800.
Guo F and Yost R S 1998 Partitioning soil phosphorus into three discrete pools of differing availability. Soil Sci. 163, 822–833.
Hagin J and Tucker B 1982 Fertilization of dry land and irrigated soils. pp. 75–90. Springer-Verlag, New York.
Hocking P J 2001 Organic acids exuded from roots in phosphorus uptake and aluminum tolerance of plants in acid soils. In Advances in Agronomy. Ed. D L Sparks. pp. 63–79. Academic Press, New York.
Holford I C R and Mattingly G E G 1975 The high and low energy phosphate absorbing surfaces in calcarreous soils. J. Soil Sci. 26, 407–417.
Hooker M L, Peterson G A, Sander D H and Diagger L A 1980 Phosphate fractions in calcareous soils as altered by time and amount of added phosphate. Soil Sci. Soc. Am. J. 44, 269–277.
International Atomic Energy Agency (IAEA) 2001 Use of isotope and radiation methods in soil and water management and crop nutrition. Training course series No. 14. FAO/IAEA Agric. and Biotech. Laboratories. IAEA, Vienna, Austria.
Ivanove A L and Shakhdzhakban M 1993 Inactivation and mobilization of phosphates in soils. Eurasian Soil Sci. (Translated) 25, 37–45.
Kuo S 1996 Phosphorus. In Methods of Soil Analysis: Part 3, Chemical Methods. Ed. D L Sparks. pp. 869–919. Soil Sci. Soc. Am., Madison, WI.
Lindsay W L 1979 Chemical Equiliberia in Soil. pp. 180–204. John Wiley and Sons, New York.
Lindsay W L, Frazier A W and Stephenson H F 1962 Identification of reaction products from phosphate fertilizers in soil. Soil Sci. Soc. Proc. 25, 446–452.
Lopez-Hernandez D, Flores D, Siegert G and Rodringuez J V 1979 The effect some organic anions on phosphate removal from acid and calcareous soils. Soil Sci. 128, 321–326.
Mattingly G E G 1971 Residual value of phosphate fertilizers on neutral and calcareous soils. Technical Bulletin 20. pp. 1–15. Ministry of Agriculture and Food, London, UK.
Morel C and Plenchette C 1994 Is the isotopically exchangeable phosphate of a loamy soil the plant-available P? Plant Soil 158, 287–297.
Olsen S R and Sommers L E 1982 Phosphorus. In Methods of Soil Analysis: Part 2, Chemical and Microbiological Properties. 2d edn. Eds. A L Page et al. pp. 403–430. Am. Soc. Agron., Madison, WI.
Olsen S R, Cole C V, Watnabe F S and Dean L A 1954 Estimation of available P in soil by extraction with sodium bicarbonate. U. S. Department of Agriculture. Circular, 939. Washington, DC.
Robert M E and Larsen S 1970 The stability of dicalcium phosphate dihydrate in soil. J. Soil Sci. 21, 353–358.
Sample E C, Soper R J and Racz G J 1980 Reactions of phosphate fertilizers in soils. In The Role of Phosphorus in Agriculture. Eds. F E Khasaweneh et al. pp. 263–310. Am. Soc. Agron., Madison, WI.
Syers J K, Smillie G W and Williams J D H 1972 Calcium flouride formation during extraction of calcareous soil with flouride: I. Implications to inorganic phosphorus fractionation schemes. Soil Sci. Soc. Am. Proc. 36, 20–24.
Tomar N K, Khanna S S and Gupta A F 1984 Transformation of mixtures of Missouri rock phosphate and TSP in calcareous soil applied after incubation with organic matter. Haryana Agriculture. Univ. J. Res. XIV, 324–333.
Wild A 1988 Plant nutrients in soil: Phosphate. In Russell's Soil Conditions & Plant Growth. Ed. A Wild. pp. 695–742. Longman Scienctific and Technical, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Asfary, A., AL-Merey, R. & Al-Hameish, M. Fractionation of applied 32P labeled TSP in calcareous soils. Plant Soil 264, 171–183 (2004). https://doi.org/10.1023/B:PLSO.0000047754.15751.6d
Issue Date:
DOI: https://doi.org/10.1023/B:PLSO.0000047754.15751.6d