Abstract
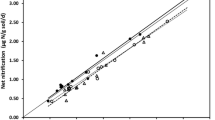
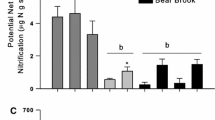
Watersheds of the Catskill Mountains, New York have marked differences in nitrogen (N) dynamics among dominant tree species stands. Our objectives were to study how tree species vary in N uptake to better understand the basis for the observed variation in these forested watersheds. We conducted a 15N tracer greenhouse study to determine NH4 + and NO3 − uptake of American beech (Fagus grandifolia Ehrh.), eastern hemlock (Tsuga Canadensis L.), red oak (Quercus rubra L.) and sugar maple (Acer saccharum Marsh.) seedlings. Seedlings and their native soil were collected in November 1997, over-wintered and allowed to break dormancy in spring 1998. Half of the seedlings of each tree species received 15NH4-NO3 to examine NH4 + uptake and the other half received NH4-15NO3 to examine NO3 − uptake. Plants were harvested 4 days following 15N addition. Tree species varied in their preference for NH4 + and NO3 −. Sugar maple and eastern hemlock seedlings took up more NH4 + than NO3 − per unit plant biomass, while beech was the only species to take up more NO3 − than NH4 +. Red oak took up more NH4 + than NO3 − into roots, stems and leaves, but the difference between the two forms of N was not statistically significant. These results demonstrate that tree species of the Catskill Mountains vary in their capacity to take up NH4 + and NO3 −. Coupled with stand-level studies of N dynamics, this variation can help explain some of the patterns of forested watershed N retention and loss in the Catskill Mountains shown in our field investigations (Templer, 2001; Templer et al., in press Ecosystems).
Similar content being viewed by others
References
Aber J, Nadelhoffer K J, Steudler P and Melillo J M 1989 Nitrogen saturation in northern forest ecosystems. BioScience 39, 378–386.
Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L and Fernandez I. 1998 Nitrogen saturation in temperate forest ecosystems. Hypotheses revisited. BioScience 48, 921–934.
Agren G I and Bosatta E 1988 Nitrogen saturation of terrestrial ecosystems. Environmental Pollution 54, 185–197.
BassiriRad H, Prior S A, Norby R J and Rogers H H 1999 A field method of determining NH+4 and NO-3 uptake kinetics in intact roots: Effects of CO2 enrichment on trees and crop species. Plant and Soil 217, 195–204.
Berntson G M and Aber J D 2000 Fast nitrate immobilization in N saturated temperate forest soils. Soil Biology and Biochemistry 32, 151–156.
Bloom A J and Caldwell R M 1988 Root excision decreases nutrient absorption and gas fluxes. Plant Physiology 87, 794–796.
Crabtree R C and Bazazz F A 1993 Tree seedling response of four birch species to simulated nitrogen deposition: Ammonium vs nitrate. Ecological Applications 3, 315–321.
Dail D B, Davidson E A and Chorover J 2000 Rapid abiotic transformation of nitrate in an acid forest soil. Biogeochemistry 54, 131–146.
Dawson T E 1993 Hydraulic lift and water use by plants: Implications for water balance, performance, and plant-plant interactions. Oecologia 95, 565–574.
Dawson T E 1996 Determining water use by trees and forests from isotopic, energy balance, and transpiration analyses: The role of tree size and hydraulic lift. Tree Physiology 16, 263–272.
Dawson T E 1998 Water loss from tree roots influences soil water and nutrient status and plant performance. InRadical Biology: Advances and Perspectives in the Function of Plant Roots. Eds. H E Flores, J P Lynch and D M Eissenstat. Current Topics in Plant Physiology, Vol. 18. pp. 235–250. American Society of Plant Physiologists, Rockville, MD, USA.
Finzi A C, Van Breemen N and Canham C D 1998 Canopy treesoil interactions within temperate forests: Species effects on soil carbon and nitrogen. Ecological Applications 8, 440–446.
Fitzhugh R D, Christenson L M and Lovett G M 2003 The fate of 15NO-2 tracer in soils under different tree species of the Catskill Mountains, New York. Soil Science Society of America Journal 67, 1257–1265.
Fitzhugh R D, Lovett G M and Venterea R T Biotic and abiotic immobilization of nitrogen in soils developed under different tree species in the Catskill Mountains, New York, USA. Global Change Biology, in press.
Freeden A L and Field C B 1992 Ammonium and nitrate uptake in gap, generalist and understory species of the genus Piper. Oecologia 92, 207–214.
Gessler A, Schneider S, von Sengbusch D, Weber P, Hanemann U, Huber C, Rothe A, Kreutzer K and Rennenberg H 1998 Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytologist 138, 275–286.
Gharbi A and Hipkin C 1984 Studies on nitrate reductase in British angiosperms. I. A comparison of nitrate reductase activity in ruderal, woodland-edge and woody species. New Phytology 97, 629–639.
Godman R M, Yawney H W and Tubbs C H 1990 Acer saccharumMarsh. Sugar Maple. InSilvics of North America, Vol. 2, Hardwoods. Eds. R M Burns and B H Honkala. Forest Service, United States Department of Agriculture, Washington, DC.
Griffin J M, Lovett G M, Arthur M A and Weathers K C 2003 The distribution and severity of beech bark disease in the Catskill Mountains, NY. Canadian Journal of Forest Research 33, 1754–1760.
Gutschick V P 1981 Evolved strategies in nitrogen acquisition by plants. American Naturalist 118, 607–637.
Horsley S B 1988 Nitrogen species preference of Prunus-serotina Ehrh. and Betula alleghaniensis Britt. Seedlings. American Journal of Botany. 1988 AnnualMeeting of the Botanical Society of America, Davis, California, USA, 14–18 August, 1988.
Houston D R, Parker E J and Lonsdale D 1979 Beech bark disease: Patterns of spread and development of the initiating agent Crptococcus fagisuga. Canadian Journal of Forest Research 9, 336–344.
Johnson D W 1992 Nitrogen retention in forest soils. Journal of Environmental Quality 21, 1–12.
Johnson D H, Cheng W and Burke I C 2000 Biotic and abiotic nitrogen retention in a variety of forest soils. Soil Science Society of America Journal 64, 1503–1514.
Knoepp J D, Turner D P and Tingey D T 1993 Effects of ammonium and nitrate on nutrient uptake and activity of nitrogen assimilating enzymes in western hemlock. Forest Ecology and Management 59, 179–191.
Larcher W 1995 Physiological Plant Ecology. Springer, Berlin.
Lovett G M and Tobiessen P 1993 Carbon and nitrogen assimilation in red oaks (Quercus rubraL.) subject to defoliation and nitrogen stress. Tree Physiology 12, 259–269.
Lovett G M and Rueth H 1999 Soil nitrogen transformations in beech and maple stands along a nitrogen deposition gradient. Ecological Applications 9, 1330–1344.
Lovett G M, Weathers K C and Sobczak W V 2000 Nitrogen saturation and retention in forested watersheds of the Catskill Mountains, New York. Ecological Applications 10, 73–84.
Lovett G M, Weathers K C and Arthur M A 2002 Control of nitrogen loss from forested watersheds by soil C:N ratio and tree species composition. Ecosystems 5, 712–718.
Lovett G M, Weathers K C, Arthur M A and Schultz J C. Nitrogen cycling in a northern hardwood forest: Do species matter? Biogeochemistry (in press).
Magill A H, Aber J D, Hendricks J J, Bowden R D, Melillo J M and Steudler P A 1997 Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecological Applications 7, 402–415.
Martin N D 1959 An analysis of forest succession in Algonquin Park, Ontario. Ecological Monographs 29, 187–217.
Min X, Siddiqi M Y, Guy R D, Glass A D M and Kronzucker H J 1998 Induction of nitrate uptake and nitrate reductase activity in trembling aspen and lodgepole pine. Plant, Cell and Environment 21, 1039–1046.
Nadelhoffer K J, Downs M R and Fry B 1999 Sinks for 15Nenriched additions to an oak forest and a red pine plantation. Ecological Applications 9, 72–86.
Ollinger S V, Aber J D, Lovett G M, Millham S E and Lathrop R G 1993 A spatial model of atmospheric deposition for the northeastern United States. Ecological Applications 3, 459–472.
Orwig D A and Foster D R 1998 Forest response to the introduced hemlock woolly adelgid in southern New England, USA. Journal of the Torrey Botanical Society 125, 60–73.
Puri G and Ashman M R 1999 Microbial immobilization of 15Nlabelled ammonium and nitrate in a temperate woodland soil. Soil Biology and Biochemistry 31, 929–931.
Recous S, Mary B and Faurie G 1990 Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biology and Biochemistry 22, 913–922.
Rennenberg H, Kreutzer K, Papen H and Weber P 1998 Consequences of high loads of nitrogen for spurce (Picea abies) and beech (Fagus sylvatica) forests. New Phytologist 139, 71–86.
Rennenberg H, Schneider S and Weber P 1996 Analysis of uptake and allocation of nitrogen and sulphur compounds by trees in the field. Journal of Experimental Botany 47, 1491–1498.
Rice C W and Tiedje J M 1989 Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biology and Biochemistry 21, 597–602.
Rothstein D E, Zak D R and Pregitzer K S 1996 Nitrate deposition in northern hardwood forests and the nitrogen metabolism of Acer saccharummarsh. Oecologia 108, 338–344.
Sander I L 1990 Quercus rubraL. Northern red oak, in Silvics of North America, Vol. 2, Hardwoods. Eds. R M Burns and B H Honkala, pp. 727–733. Forest Service United States Department of Agriculture, Washington, DC.
Sarjala T 1991 Effects of mycorrhiza and nitrate nutrition on nitrate reductase activity in Scots pine seedlings. Physiologia Plantarum 81, 89–94.
Stark J M and Hart S C 1996 Diffusion technique for preparing salt solutions, Kjeldhal digests, and persulfate digests for nitrogen-15 analysis. Soil Science Society of America Journal 60(6), 1846–1855.
Stewart G R, Joly C A and Smirnoff N 1992 Partitioning of inorganic nitrogen assimilation between the roots and shoots of cerrado and forest trees of contrasting plant communities of South East Brasil. Oecologia 91, 511–517.
Stoddard J L 1994 Long-term changes in watershed retention of nitrogen. InEnvironmental Chemistry of Lakes and Reser voirs, Advance in Chemistry Series, Vol. 237, Ed. L A Baker, pp. 223–284. American Chemical Society, Washington, DC.
Templer P H 2001 Direct and indirect effects of tree species on forest nitrogen retention in the Catskill Mountains, NY. PhD Dissertation, Cornell University.
Templer P, Findlay S and Lovett G 2003 Soil microbial biomass and nitrogen transformations among five tree species of the Catskill Mountains, New York, USA. Soil Biology and Biochemistry 35, 607–613.
Templer P H, Lovett G, Weathers K, Findlay S and Dawson T In-fluence of tree species on forest nitrogen retention in the Catskill Mountains, New York, USA. Ecosystems (in press).
Tischner R 2000 Nitrate uptake and reduction in higher and lower plants. Plant, Cell and Environment 23, 1005–1024.
Tubbs C H and Houston D R 1990 Fagaceae Beech Family, InSilvics of North America. Vol. 2, Hardwoods, Eds. R M Burns and B H Honkala, pp. 325–332. Forest Service, United States Department of Agriculture, Washington, DC.
Vitousek P M 1994 Beyond global warming: Ecology and global change. Ecology 75, 1861–1876.
Wickramasinghe K N, Rodgers G A and Jenkinson D S 1985 Transformations of nitrogen fertilizers in soil. Soil Biology and Biochemistry 17, 625–630.
Zak D R and Pregitzer K S 1990 Spatial and temporal variability of nitrogen cycling in Northern lower Michigan. Forest Science 36, 367–380.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Templer, P., Dawson, T. Nitrogen uptake by four tree species of the Catskill Mountains, New York: Implications for forest N dynamics. Plant and Soil 262, 251–261 (2004). https://doi.org/10.1023/B:PLSO.0000037047.16616.98
Issue Date:
DOI: https://doi.org/10.1023/B:PLSO.0000037047.16616.98