Abstract
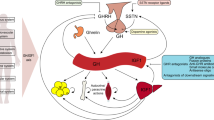
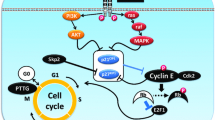
Pituitary tumors cause considerable morbidity due to local invasion, hypopituitarism, or hormone hypersecretion. In many cases, no suitable drug therapies are available, and surgical excision is currently the only effective treatment. We have recently demonstrated abundant expression of nuclear hormone receptor PPAR-γ in human pituitary tumors of different subtypes. PPAR-γ activators (thiazolidinediones) induced G0-G1 cell-cycle arrest and apoptosis in human, and murine corticotroph, somatolactotroph, and gonadotroph pituitary tumor cells, and suppressed in vitro hormone secretion. In vivo development and growth of murine corticotroph, somatolactotroph and gonadotroph tumors, generated by subcutaneous injection of ACTH-secreting AtT20, PRL- and GH-secreting GH3, and LH-secreting LβT2, and α-T3 cells, was markedly suppressed in rosiglitazone treated mice, and plasma ACTH, and serum corticosterone, GH, PRL and LH levels were attenuated in all treated animals. PPAR-γ is an important novel molecular target in pituitary adenoma cells and as PPAR-γ ligands inhibit tumor cell growth and ACTH, GH, PRL and LH secretion in vitro and in vivo, thiazolidinediones are proposed as a novel oral medical management for pituitary tumors.
Similar content being viewed by others
References
Heaney AP, Melmed S. Molecular pathogenesis of pituitary tumors. In: Wass JAH, Shalet SM, eds. Oxford Textbook of Endocrinology. Oxford: Oxford University Press, 2002;2:109–120.
Central Brain Tumor Registry of the United States (CBTRUS). http://www.cbtrus.org.
Sano K. Incidence of primary tumors (1969–1983). In: Brain Tumor Registry of Japan. Neurol Med Chir 37(special issue)1992:391–441.
Shimon I, Melmed S. Management of pituitary tumors. Ann Intern Med 1998;129:472–483.
Freda PU, Wardlaw SL. Primary medical therapy for acromegaly. J Clin Endocriol Metab 1998;83:3031–3033.
Newman CB, Melmed S, George A, Torigan D, Duhaney M, Snyder P et al. Safety and efficacy of long-term octreotide therapy of acromegaly: Results of a multicenter trial in 103 patients—a clinical research center study. J Clin Endocrinol Metab 1995;80:2768–2775.
Vance ML, Harris AG. Long-term treatment of 189 acromegalic patients with the somatostatin analog octreotide. Results of a multicenter acromegaly study group. Arch Int Med 1991;151:1573–1578.
Colao A, Ferone D, Marzullo P, Cappabianca P, Cirillo S, Boerlin V, Lancranjan I, Lombardi G. Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab 2001;86:2779–2786.
Baldelli R, Colao A, Razzore P, Jaffrain-Rea ML, Marzullo P, Ciccarelli E, Ferretti E, Ferone D, Gaia D, Camanni F, Lombardi G, Tamburrano G. Two-year follow-up of acromegalic patients treated with slow release lanreotide (30 mg). J Clin Endocrinol Metab 2000;85:4099–4103.
Freda PU. Somatostatin analogs in acromegaly. J Clin Endocrinol Metab 2002;87:3013–3018.
Melmed S, Casanueva FF, Cavagini F, Chanson P, Frohman L, Glossman A, Ho K, Kleinberg D, Lamberts S, Laws E, Lombardi G, Vance ML, Von Werder K, Wass J, Giustina A. Consensus: Guidelines for acromegaly management. J Clin Endocrinol Metab 2001;87:4054–4058.
Sheppard MC. Primary medical therapy for acromegaly. Clin Endocrinol (Oxf) 2003;58:387–399.
Molitch ME. Medical management of prolactin-secreting pituitary adenomas. Pituitary 2002;5:55–65.
Klibanski A, Zervas NT. Diagnosis and management of hormone-secreting pituitary adenomas. N Eng J Med 1991;324: 822–831.
Bevan JS, Webster, Burke CW, Scanlon MF. Dopamine agonists and pituitary tumor shrinkage. Endocr Rev 1992;3:220–240.
Kleinberg DL, Boyd AE 3rd, Wardlaw S, Frantz AG, George A, Bryan N et al. Pergolide for the treatment of pituitary tumors secreting prolactin or growth hormone. N Eng J Med 1982;309:704–709.
Vance ML, Lipper M, Klibanski A, Biller BM, Samaan NA, Molitch ME. Treatment of prolactin-secreting pituitary macroadenomas with the long-acting non-ergot dopamine agonist CV 205–502. Ann Intern Med 1990;112:668–673.
Colao A, Di Sarno A, Sarnacchiaro F, Ferone D, Di Renzo G, Merola B et al. Prolactinomas resistant to standard dopamine agonists respond to chronic cabergoline treatment. J Clin Endocrinol Metab 1997;82:876–883.
Webster J, Piscitelli G, Polli A, Ferri CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med 1994;331:904–909.
Orth DN. Cushing's syndrome. N Engl J Med 1995;332:791–803.
Findling JW, Raff H. Diagnosis and differential diagnosis of Cushing's syndrome. Endocrinol Metab Clin North Am 2001;30:729–747.
Mampalam TJ, Tyrrell JB, Wilson CB. Transsphenoidal microsurgery for Cushing's disease: A report of 216 cases. Ann Intern Med 1988;109:487–493.
Simmons NE, Alden TD, Thorner MO, Laws ER Jr. Serum cortisol response to transphenoidal surgery for Cushing disease. J Neurosurg 2001;95:1–8.
Trainer PJ et al. Transsphenoidal resection in Cushing's disease: Undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol 1993;8:73–78.
Hoybye C et al. Adrenocorticotrophic hormone-producing pituitary tumors: 12 to 22-year follow-up after treatment with sterotactic radiosurgery. Neurosurgery 2001;49:284–291.
Trainer PJ, Besser M. Cushing's syndrome: Therapy directed at the adrenal glands. Endocrino Metab Clin North Am 1994;23:571–584.
Brada M et al. The long-tern efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol 1993;38:571–578.
Estrada J, Boronat M, Mielgo M, Magallon R, Millan I, Diez S, Lucas T, Barcelo B. The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing's disease. N Engl J Med 1997;336:172–177.
Erfurth EM, Bulow B, Mikoczy Z, Hagmar L. Incidence of a second tumor in hypopituitary patients operated for pituitary tumors. J Clin Endocrinol Metab 2001;86:659–662.
Niemann LK. Medical therapy of Cushing's disease. Pituitary 2002;5:77–82.
Morris D, Grossman A. The medical management of cushing's syndrome. Ann N Y Acad Sci 2002;970:119–133.
Kennedy AL, Sheridan B, Montgomery DAD. ACTH and cortisol response to bromocriptine and results of long-term therapy in Cushing's disease. Acta Endocrinol (Copenh) 1978;89:461–468.
Muller OA, Fahlbusch R, Horowski R. Effect of lisuride on ACTH levels in patients with active Cushing's disease. Acta Endocrinol (Copenh) 1982;99[Suppl 246]:l03–104.
Boscaro M, Benato M, Mantero P. Effect of bromocriptine in pituitary dependent Cushing's syndrome. Clin Endocrinol (Oxf) 1983;19:485–491.
Verlhest JA, Trainer PJ, Howlett TA et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol 1991;35:169–178.
Tabarin A, Navarranne A, Guerin J, Corcuff JB, Parmiex M, Roger P. Use of ketaconazole in the treatment of Cushing's diaease and ectopic ACTH syndrome. Clin Endocrinol 1991;34:63–69.
Sonino N, Boscaro M, Merola G, Mantero F. Prolonged treatment of Cushing's disease by ketoconazole. J Clin Endocrinol Metab 1985;61:718–722.
Boscaro M, Sonino N, Rampazzo A, Mantero F. Response of pituitary-adrenal axis to corticotrophin releasing hormone in patients with Cushing's disease before and after ketoconazole treatment. Clin Endocrinol 1987;27:461–467.
McCance DR, Ritchie CM, Sheridan H, Atkinson AB. Acute hypoadrenalism and hepatotoxicity after treatment with ketoconazole. Lancet 1981;1:573.
Bertagna X, Bertagna C, Laudat MH, Husson J-M, Girard F, Luton JP. Pituitary-adrenal response to the antiglucocorticoid action of RU 486 in Cushing's syndrome. J Clin Endocrinol Metab 1986;63:639–643.
Nieman LK, Chrousos GP, Kellner C et al. Successful treatment of Cushing's syndrome with the glucocorticoid antagonist RU-486. J Clin Endocrinol Metab 1985; 61:536–550.
Greenman Y, Melmed S. Diagnosis and management of nonfunctioning pituitary tumors. Ann Rev of Med 1996;7:95–106.
Ebersold MJ, Quast LM, Laws ER, Scheithauer B, Randall RV. Long-term results in transsphenoidal removal of nonfunctioning pituitary adenomas. J Neurosurg 1986;64:713–719.
Nobels FR, de Herder WW, van den Brink WM, Kwekkeboom DJ, Hofland LJ, Zuyderwijk J, de Jong FH, Lamberts SW. Longterm treatment with the dopamine agonist quinagolide of patients with clinically non-functioning pituitary adenoma. Eur J Endocrinol 2000;143:615–621.
Bevan JS, Burk CW. Non-functioning pituitary adenomas do not regress during bromocriptine therapy but possess membrane-bound dopamine receptors, which bind bromocriptine. Clin Endocrinol 1986;25:561–572.
Freda PU, Wardlaw SL. Diagnosis and treatment of pituitary tumors. J Clin Endocrinol Metab 1999;84:3859–3866.
Lemberger T, Desverge B, Wahli W. Peroxisome proliferators-acitvated receptors: A nuclear receptor signaling pathway in lipid physiology. Ann Rev Cell Dev Biol 1996;12:355–363.
Mangelsforf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell 1996;83:841–850.
Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions for peroxisome proliferators. Annu Rev Pharmacol Toxicol 2000;40:491–518.
Kodera Y, Takeyama KI, Murayama A, Suzawa M, Masuhiro Y, Kato S. Ligand-type specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. J Biol Chem 2000;275:33201–33201.
Wu GD, Lazar MA. A gut check for PPAR γ. Gastroenterology 1998;115:1283–1285.
Vidal-Puig A, Jimenez-Lanan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Blier JS, Moller DE. Regulation of PPAR γ gene expression by nutrition and obesity in rodents. J Clin Investig 1996;97:2553–2561.
Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruehart JC, Deeb S, Vidal-Puig A, Flier J, Briggs JR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis and expression of the human PPAR γ gene. J Biol Chem 1997;272:18779–18789.
DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor PPAR γ is aberrantly expressed in colonic cancers. Carcinogenesis (Lond) 1998;19:49–53.
Parks KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, Henry RR. PPAR γ gene expression is elevated in skeletal muscle of obese and type II diabetic subjects. Diabetes 1997;46:1230–1234.
Mueller E, Sarraf P, Tontonoz P, Evans RM, Markin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM. Terminal differentiation of human breast cancer through PPAR γ. Mol Cell 1998;1:465–470.
Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Mave RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu G, Dannenberg AJ, Flanders KD, Letterio RF, Roberts AB, Rohe NS, Subbaramaiah K, Sporn MB. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res 1999;59:336–341.
Wang Y, Porter WW, Suh N, Honda T, Gribble GW, Leesnitzer LM, Plunket KD, Mangelsorf DJ, Blanchard SG, Wilson TM, Sporn MB. A synthetic triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (DDDO), is a ligand for the perxoisome proliferator-activated receptor γ. Mol Endocrinol 2000;14:1550–1556.
Chawla A, Baak Y, Nagy L, Lio D, Tontonoz P, Evans RM. PPAR γ dependent and independent effects on macrophagegene expression in lipid metabolism. Nat Med 2001;1:48–52.
Jiang C, Ting AT, Seed B. PPAR γ agonists inhibit production of monocyte inflammatory cytokines. Naute (Lond) 1998;391:82–86.
Li M, Pascual G, Glass CK. Peroxisome proliferator-activate receptor γ-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol 2000;20:4699–4707.
Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, Libby R, Plutzky J, Luster AD. Peroxisome proliferators-activated receptor-γ activators inhibit IFN-γ-induced expression of the T-cell active CXC chemokines IPI0, Mig, and I-TAT in human endothelial cells. J Immunol 2000;164: 6504–6508.
Ricote J, Li AC, Wilson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor γ is a negative regulator of macrophage activation. Nature (Lond) 1998;39:79–82.
Warrell RP, Frankel SR, Miller WH, Scheinberg DA, Itri LM, Hittelman WN, Vyas R, Andreeff M, Tafuri A, Jakubowski A. Differentiation therapy of acute promyeolocytic leukemia with tretinoin (all trans-retionoic-acid). N Eng J Med 1991;324:1385–1393.
Warrell R, de The H, Wang ZY, Degos LN. Acute promyelocytic leukemia. New Eng J Med 1992;329:177–189.
Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CE, Brun RP, Mueller E, Altiok S, Oppenheim H, Evans RM, Spiegelman BM. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor and the retinoid X receptor. Proc Natl Acad Sci USA 1997;94:237–241.
Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA 1999;96:3951–3956.
Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Deuchand P, Wahli W, Wilson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids proliferators-activated receptors and α and γ. Proc Natl Acad Sci USA 1997;94:4318–4323.
Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of enteric-coated fish-oil preparation on relapses in Crohn's disease. N Eng J Med 1996;334:1447–1560.
Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, Aaltonen LA, de la Chapelle A, Spiegelman BM, Eng C. Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol Cell 1999;3:799–804.
Ikezoe T, Miller CW, Kawano S, Heaney A, Williamson EA, Hisatake J, Green E, Hofmann W, Taguchi H, Koeffler HP. Mutational analysis of the peroxisome proliferator-activated receptor gamma gene in human malignancies. Cancer Res 2001;1:61:5307–5310.
Girnum G, Sarraf P, Mueller E, Drori S, Gonzalez F, Spiegelman B. The role of PPAR γ as a tumor suppressor gene in colon carcinogenesis. Keystone Symposia: The PPARs: A transcription odyssey (Abstract) 2001;73.
Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPAR γ enhance colon polyp formation. Nat Med 1998;4:1053–1057.
Lefebvre A, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/mice. Nat Med 1998;4:10531–10557.
Girnun GD, Smith WM, Drori S, Sarraf P, Mueller E, Eng C, Nambiar P, Rosenberg DW, Bronson RT, Edelman W et al. APCdependent suppression of colon carcinogenesis by PPAR γ. Proc Natl Acad Sci 2002;99:13771–13776.
Joyce D, Albanese C, Pestell RG. NF-κβ and cell-cycle regulation: The cyclin connection. Cytokin Growth Factor Rev 2001;12:73–90.
Karin M, Delhase M. The Iκβ (IKK) and NF-κβ: Key elements of proinflammatory signaling. Semin Immunol 2000;12:85–89.
Gupta RA, Brockman JA, Sarraf O, Willson TM, DuBois RN. Target genes of peroxisome proliferator-activated receptor γ in colorectal cancer cells. J Biol Chem 2001;276:29681–29867.
Heaney AP, Fernando M, Yong W, Melmed S. Functional PPAR-γ receptor represents a novel therapeutic target in Cushing's disease. Nat Med 2002;11:1281–1287.
Heaney AP, Fernando M, Melmed S. PPAR-γ receptor ligands: A novel therapy for pituitary tumors. J Clin Invest 2003;111:1381–1388.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heaney, A.P. Novel Pituitary Ligands: Peroxisome Proliferator Activating Receptor-γ. Pituitary 6, 153–159 (2003). https://doi.org/10.1023/B:PITU.0000011176.05771.46
Issue Date:
DOI: https://doi.org/10.1023/B:PITU.0000011176.05771.46