Abstract
Purpose. The Caco-2 association of lectin-grafted PLGA-nanospheres was investigated compared to plain and BSA-coated spheres.
Methods. Nanospheres made from fluorescent-labeled PLGA were coated with wheat germ agglutinin (WGA) or BSA and incubated with Caco-2 monolayers varying the concentration of nanospheres, the time, and the temperature. The tests were performed in a static horizontal as well as an aerated vertical setup to find out the system most appropriate for estimation of bioadhesion.
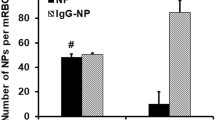
Results. Due to bioadhesive effects, WGA-modified particles exhibited highest association to the cells as compared to plain and BSA-coated ones. The amount of associated spheres increased with time and concentration of the nanosphere suspension. Whereas the binding of lectin-coated spheres was independent from energy, their uptake was energy consuming as opposed to BSA and plain nanospheres, which exhibited nonspecific, energy independent binding and uptake. Although more particles were associated with the monolayer in the horizontal setup than in the vertical system, the vertical system reflects true bioadhesion due to circulation of the spheres which inhibits the influence of sedimentation.
Conclusions. Immobilization of WGA considerably enhances the binding as well as the uptake of PLGA-nanospheres by Caco-2 monolayers. For bioadhesion studies, the vertical setup is recommended instead of the horizontal setup.
Similar content being viewed by others
REFERENCES
A. T. Florence. The oral absotprion of micro-and nanoparticu-lates: Neither exceptional nor unusual. Pharm. Res. 14:259–266 (1997).
A. A. Ignatius and L. E. Claes. In vitrobiocompatibility of bio-resorbable polymers: poly(L,DL-lactide) and poly(L-lactide-co-glycolide). Biomaterials 17:831–839h (1996).
H. R. Lin, C. J. Kuo, C. Y. Yang, S. Y. Shaw, and Y. J. Wu. Preparation of macroporous biodegradable PLGA scaffolds for cell attachment with the use of mixed salts as porogen additives. J. Biomed. Mater. Res. 63:271–279 (2002).
J. Panyam and V. Labhasetwar. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Del. Rev. 55:329-347 (2003).
J. M. Anderson and M. S. Shive. Biodegradation and biocompatibility of PLA and PLGA microspheres Adv. Drug Del.Rev. 28:5–24 (1997).
G. J. Russell-Jones, H. Veitch, and L. Arthur. Lectin mediated transport of nanoparticles across Caco 2 and OK cells. Int. J. Pharm. 190:165–174 (1999).
M. P. Desai, V. Labhasetwar, E. Walter, R. J. Levy, and G. L. Amidon. The mechanism of uptake of biodegradable micropar-ticles in Caco-2 cells is size dependent. Pharm. Res. 14:1568–1573 (1997).
S. McClean, E. Prosser, and E. Meehan. D. ÓMalley, N. Clarke, Z. Ramtoola, and D. Brayden. Binding and uptake of biodegrad-able poly-DL-lactide micro-and nanoparticles in intestinal epi-thelia. Eur. J. Pharm. Sci. 6:153–163 (1998).
N. Hussain, P. U. Jani, and A. T. Florence. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm. Res. 14:613-618 (1997).
E. Mathiowitz, J. S. Jacob, Y. S. Jong, G. P. Carino, D. E. Chick-ering, P. Chaturvedi, C. A. Santos, K. Vijayaraghavan, S. Mont-gomery, M. Bassett, and C. Morrell. Biologically erodable micro-spheres as potential oral drug delivery systems. Nature 386:410–414 (1997).
F. Gabor, M. Wirth, B. Jurkovich, I. Haberl, G. Theyer, G. Walcher, and G. Hamilton. Lectin-mediated bioadhesion: Pro-teolytic stability and binding-characteristics of wheat germagglu-tinin and Solanum tuberosumlectin on Caco-2, HT-29 and human colonocytes. J. Control. Rel. 49:27–37 (1997).
M. Wirth, C. Kneuer, C. M. Lehr, and F. Gabor. Lectin mediated drug delivery: discrimination between cytoadhesion and cytoin-vasion and evidence for lysosomal accumulation of wheat germ agglutinin in the Caco 3 model. J. Drug Targ. 10:439–448 (2002).
B. Ertl, F. Heigl, M. Wirth, and F. Gabor. Lectin-mediated bio-adhesion: preparation, stability and Caco 2 binding of wheat germ agglutinin-functionalized poly(D,L lactic co glycolic acid) microspheres. J. Drug Targ. 8:173–184 (2000).
B. Ertl, P. Platzer, M. Wirth, and F. Gabor. Poly(D,L-lactic-co-glycolic acid) microspheres for sustained delivery and stabiliza-tion of camptothecin. J. Control. Rel. 61:305–317 (1999).
G. J. Russell-Jones, L. Arthur, and H. Walker. Vitamin B12-mediated transport of nanoparticles across Caco-2 cells. Int. J. Pharm. 179:247–255 (1999).
Y. Li, M. Ogris, E. Wagner, J. Pelisek, and M. Rueffer. Nanoparticles bearing polyethylenglycol coupled transferrin as gene carriers: preparation and in vitro evaluation Int. J. Pharm. 259:93–101 (2003).
Y. Zhang, N. Kohler, and M. Zhang. Surface modification of superparamagnetic magnetite nanoparticles and their intracellu-lar uptake. Biomaterials 23:1553–1561 (2002).
N. Hussain, P. U. Jani, and A. T. Florence. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm. Res. 14:613–618 (1997).
V. Labhetswar, C. Song, W. Humphrey, R. Shebuski, and R. J. Levy. Arterial uptake of biodegradable nanoparticles: Effect of surface modifications. J. Pharm. Sci. 87:1229–1234 (1998).
J. V. Staros, R. W. Wright, and D. M. Swingle. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156:220–222 (1986).
D. G. Hoare and D. E. Koshland Jr. A Method for the Quanti-tative Modification and Estimation of Carboxylic Acid Groups in Proteins. J. Biol. Chem. 242:2447–2453 (1967).
T. Jung, W. Kamm, A. Breitenbach, E. Kaiserling, J. X. Xiao, and T. Kissel. Biodegradable nanoparticles for oral delivery of pep-tides: is there a role for polymers to affect mucosal uptake? Eur. J. Pharm. Biopharm. 50:147–160 (2000).
M. P. Desai, V. Labhasetwar, G. Amidon, and R. J. Levy. Gas-trointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 13:1838–1845 (1996).
D. E. Chickering III, C. A. Santos, and E. Mathiowitz. Adaption of a microbalance to measure bioadhesive properties of micro-spheres. In E. Mathiowitz, D.E. Chickering III, and C.M. Lehr (eds.), Bioadhesive Drug Delivery Systems, Marcel Dekker, New York, 1999, pp. 131–146.
B. A. Hertzog and E. Mathiowitz. Novel magnetic technique to measure bioadhesion. In E. Mathiowitz, D.E. Chickering III, and C.M. Lehr (eds.), Bioadhesive Drug Delivery Systems, Marcel Dekker, New York, 1999, pp. 147–173.
N. Lochner, F. Pittner, M. Wirth, and F. Gabor. Wheat germ agglutinin binds to the epidermal growth factor of artificial Caco-2 membranes as detected by silver nanoparticle enhanced fluorescence. Pharm. Res. 20:833–839 (2003).
F. Gabor, E. Bogner, A. Weissenboeck, and M. Wirth. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Del. Rev. 56:459–480 (2004).
F. Gabor, A. Schwarzbauer, and M. Wirth. Lectin-mediated drug delivery: Binding and uptake of BSA-WGA conjugates using the Caco-2 model. Int. J. Pharm. 237:227–239 (2002).
J. Panyam, W. Zhou, S. Prabha, S. K. Sahoo, and V. Labhasetwar. Rapid endo-lysosomal escape of poly(D,L-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 16:1217-1226 (2002).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weissenboeck, A., Bogner, E., Wirth, M. et al. Binding and Uptake of Wheat Germ Agglutinin-Grafted PLGA-Nanospheres by Caco-2 Monolayers. Pharm Res 21, 1917–1923 (2004). https://doi.org/10.1023/B:PHAM.0000045247.09724.26
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000045247.09724.26