Abstract
Purpose. The purpose of this study was to assess the influence of nonlinear renal clearance on the ability of urinary excretion data to accurately determine relative differences in systemic exposure and bioavailability.
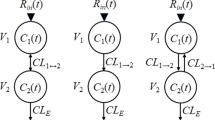
Methods. Serum concentration and urinary excretion-time profiles were simulated assuming an open one-compartmental model with first-order absorption, linear nonrenal clearance, and either linear or nonlinear renal clearance (saturable secretion). Renal clearance comprised 5% or 95% of total clearance. Doses were varied over a 100-fold range (10-fold decrease/increase from the reference dose). Relative systemic exposures were based on the ratios of AUC and Cmax and the corresponding ratios of cumulative amount excreted in urine (Ae) and the maximum urinary excretion rate. Relative bioavailability was based on the ratios of Ae and the test to reference dose (Dratio).
Results. When renal clearance was linear and urinary excretion data were used to assess relative systemic exposure and relative bioavailability, no significant errors in accuracy were observed. However, when renal clearance was nonlinear, errors in the accuracy of estimation of relative bioavailability (Clr =5% only) and relative systemic exposure ranged from -53% to +125%; minimal error in accuracy existed in the estimation of relative bioavailability when Clr = 95% (−3% to +6%).
Conclusions. Prior to the use of urinary excretion data to assess relative systemic exposure or bioavailability, the relationship between serum concentration and renal clearance should be established.
Similar content being viewed by others
references
Food and Drug Administration. Bioavailability and bioequivalence requirements. Fed. Regist. 42:1624–1653 (1977).
W. J. Westlake. Use of statistical methods in evaluation of in vivo performance of dosage forms. J. Pharm. Sci. 62:1579–1589 (1973).
W. J. Westlake. Use of confidence intervals in analysis of comparative bioavailability trials. J. Pharm. Sci. 61:1340–1341 (1972).
E. Purich. Bioavailability/Bioequivalence Regulations: An FDA Perspective. In K. S. Albert (ed.), Drug Absorption and Disposition: Statistical Considerations, American Pharmaceutical Association Academy of Pharmaceutical Sciences, Washington, DC, 1980, pp. 115–137.
T. W. Dobbins and B. Thiyagarajan. A retrospective assessment of the 75/75 rule in bioequivalence. Stat. Med. 11:1333–1342 (1992).
D. J. Schuirmann. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacokinet. Biopharm. 15:657–680 (1987).
K. K. Midha, E. D. Ormsby, J. W. Hubbard, G. McKay, E. M. Hawes, L. Gavalas, and I. J. McGilveray. Logarithmic transformation in bioequivalence—application with 2 formulations of perphenazine. J. Pharm. Sci. 82:138–144 (1993).
W. W. Hauck and S. Anderson. Types of bioequivalence and related statistical considerations. Int. J. Clin. Pharm. Ther. Toxicol. 30:181–187 (1992).
L. B. Sheiner. Bioequivalence revisited. Stat. Med. 11:1777–1788 (1992).
J. G. Zhi. Initial slope technique to estimate first-and zero-order drug absorption rate constants. J. Pharm. Sci. 77:816–817 (1988).
H. Cheng, A. E. Staubus, and L. Shum. An area function method for estimating the apparent absorption rate constant. Pharm. Res. 5:57–60 (1988).
R. P. Basson, B. J. Cerimele, K. A. Desante, and D. C. Howey. Tmax: an unconfounded metric for rate of absorption in single dose bioequivalence studies. Pharm. Res. 13:324–328 (1996).
L. Endrenyi, S. Fritsch, and W. Yan. Cmax/AUC is a clearer measure than Cmax for absorption rates in investigations of bioequivalence. Int. J. Clin. Pharm. Ther. Toxicol. 29:394–399 (1991).
A. Rostamihodjegan, P. R. Jackson, and G. T. Tucker. Sensitivity of indirect metrics for assessing “rate” in bioequivalence studies-moving the “goalposts” or changing the “game”. J. Pharm. Sci. 83:1554–1557 (1994).
M. L. Chen, V. Shah, R. Patnaik, W. Adams, A. Hussain, D. Conner, M. Mehta, H. Malinowski, J. Lazor, S. M. Huang, D. Hare, L. Lesko, D. Sporn, and R. Williams. Bioavailability and bioequivalence: An FDA regulatory overview. Pharm. Res. 18:1645–1650 (2001).
Food and Drug Administration. Bioavailability and bioequivalence studies for orally administered drug product: general considerations. Available at: www.fda.gov/cder/guidance/index.htm (2003)
Food and Drug Administration. Exposure-response relationships—study design, data analysis, and regulatory applications. Available at: www.fda.gov/cder/guidance/index.htm (2003)
M. Gibaldi and D. Perrier. Pharmacokinetics, Marcel Dekker, Inc., New York, 1982.
P. L. Bonate, K. Reith, and S. Weir. Drug interactions at the renal level: Implications for drug development. Clin. Pharmacokinet. 34:375–404 (1998).
J. V. S. Gobburu and P. J. Marroum. Utilisation of pharmacokinetic-pharmacodynamic modelling and simulation in regulatory decision-making. Clin. Pharmacokinet. 40:883–892 (2001).
B. J. Gertz, S. H. Holland, W. F. Kline, B. K. Matuszewski, A. Freeman, H. Quan, K. C. Lasseter, J. C. Mucklow, and A. G. Porras. Studies of the oral bioavailability of alendronate. Clin. Pharmacol. Ther. 58:288–298 (1995).
A. G. Porras, S. D. Holland, and B. J. Gertz. Pharmacokinetics of alendronate. Clin. Pharmacokinet. 36:315–328 (1999).
D. Y. Mitchell, M. A. Heise, K. A. Pallone, M. E. Clay, J. D. Nesbitt, D. A. Russell, and C. W. Melson. The effect of dosing regimen on the pharmacokinetics of risedronate. Brit. J. Clin. Pharmacol. 48:536–542 (1999).
D. Y. Mitchell, R. A. Eusebio, N. A. Sacco-Gibson, K. A. Pallone, S. C. Kelly, J. D. Nesbitt, C. P. Brezovic, G. A. Thompson, and J. H. Powell. Dose-proportional pharmacokinetics of risedronate on single-dose oral administration to healthy volunteers. J. Clin. Pharmacol. 40:258–265 (2000).
G. A. Thompson, J. H. Powell, and S. Mallikaarjun. Bisphosphonate renal clearance: Assessment of linearity. Pharm. Res. 14:S-510(1997).
K. Besseghir and F. Roch-Ramel. Renal excretion of drugs and other xenobiotics. Renal Physiol. 10:221–241 (1987).
W. Weber, M. Nitz, and M. Looby. Nonlinear kinetics of the thiamine cation in humans: Saturation of nonrenal clearance and tubular reabsorption. J. Pharmacokinet. Biopharm. 18:501–523 (1990).
W. Weber, S. Toussaint, M. Looby, M. Nitz, and H. Kewitz. System analysis in multiple dose kinetics: Evidence for saturable tubular reabsorption of the organic cation N1-methylnicotinamide in humans. J. Pharmacokinet. Biopharm. 19:553–574 (1990).
C. A. M. Van Ginneken and F. G. M. Russel. Saturable pharmacokinetics in the renal excretion of drugs. Clin. Pharmacokinet. 16:38–54 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, G.A., Toothaker, R.D. Urinary Excretion: Does It Accurately Reflect Relative Differences in Bioavailability/Systemic Exposure When Renal Clearance Is Nonlinear?. Pharm Res 21, 781–784 (2004). https://doi.org/10.1023/B:PHAM.0000026428.48103.4f
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000026428.48103.4f